Sunday Poster Session
Category: IBD
P1123 - Uncovering Drivers of Endoscopic Monitoring Before Treatment Changes in Inflammatory Bowel Disease
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Katherine L. Stone, MD
NYU Langone Health
New York, NY
Presenting Author(s)
Katherine L.. Stone, MD1, Xucong Meng, MPH2, Jordan Axelrad, MD, MPH3, Aasma Shaukat, MD, MPH, FACG4, Adam Faye, MD, MS1
1NYU Langone Health, New York, NY; 2NYU Grossman School of Medicine, New York, NY; 3Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 4NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY
Introduction: Since the publication of the 2015 STRIDE guidelines, the management of inflammatory bowel disease (IBD) has increasingly adopted a treat-to-target (T2T) approach, emphasizing the achievement of endoscopic remission. Despite these recommendations, fewer than half of patients undergo follow-up endoscopy after initiating therapy. To better understand current clinical practice, we aimed to evaluate how often objective assessments (e.g., endoscopy, imaging, biomarkers) are used to guide treatment decisions, particularly among patients who have been on an advanced therapy for at least six months but subsequently undergo a change in therapy.
Methods: We performed a retrospective analysis of IBD patients (≥ 18 years) at an academic center who began a new advanced therapy between Jan 1, 2016, and May 31, 2023, and switched therapies after ≥ 6 months. Patients with prior colectomy were excluded. Demographics and clinical data were extracted from electronic medical records. Logistic regression identified factors associated with colonoscopy within 3 months before therapy change.
Results: Of 1,169 patients (519 with ulcerative colitis, 642 with Crohn’s disease, and 8 with indeterminate colitis), only 265 (23%) had colonoscopy within 3 months before switching therapies. Among the 904 patients who did not have a colonoscopy, 537 (59%) underwent lab monitoring (CRP, calprotectin, lactoferrin) and 79 (9%) had imaging (CT, MRI, ultrasound). Male sex was associated with higher odds of colonoscopy (aOR 1.47, 95% CI 1.11–1.97). Prior healthcare engagement, including a prior T2T colonoscopy (i.e., within 3-15 months of the original therapy) (aOR 2.33, 95% CI 1.74–3.12), recent gastroenterology visits (aOR 2.15, 95% CI 1.61-2.88), and non-endoscopic monitoring through labs and imaging as defined above (aOR 1.47, 95% CI 1.06-2.05), were also linked to greater likelihood of undergoing endoscopic assessment. However, the type of therapy being initiated (e.g., TNF inhibitor, other biologic, or small molecule) was not associated with differences in endoscopy use.
Discussion: Fewer than one-third of patients completed endoscopic or imaging assessments before switching advanced therapies. Prior engagement in care—especially successful T2T monitoring—was the strongest predictor of endoscopic evaluation. These findings may inform future efforts to identify and address barriers to objective monitoring, and support interventions to promote timely disease assessment before therapy changes.
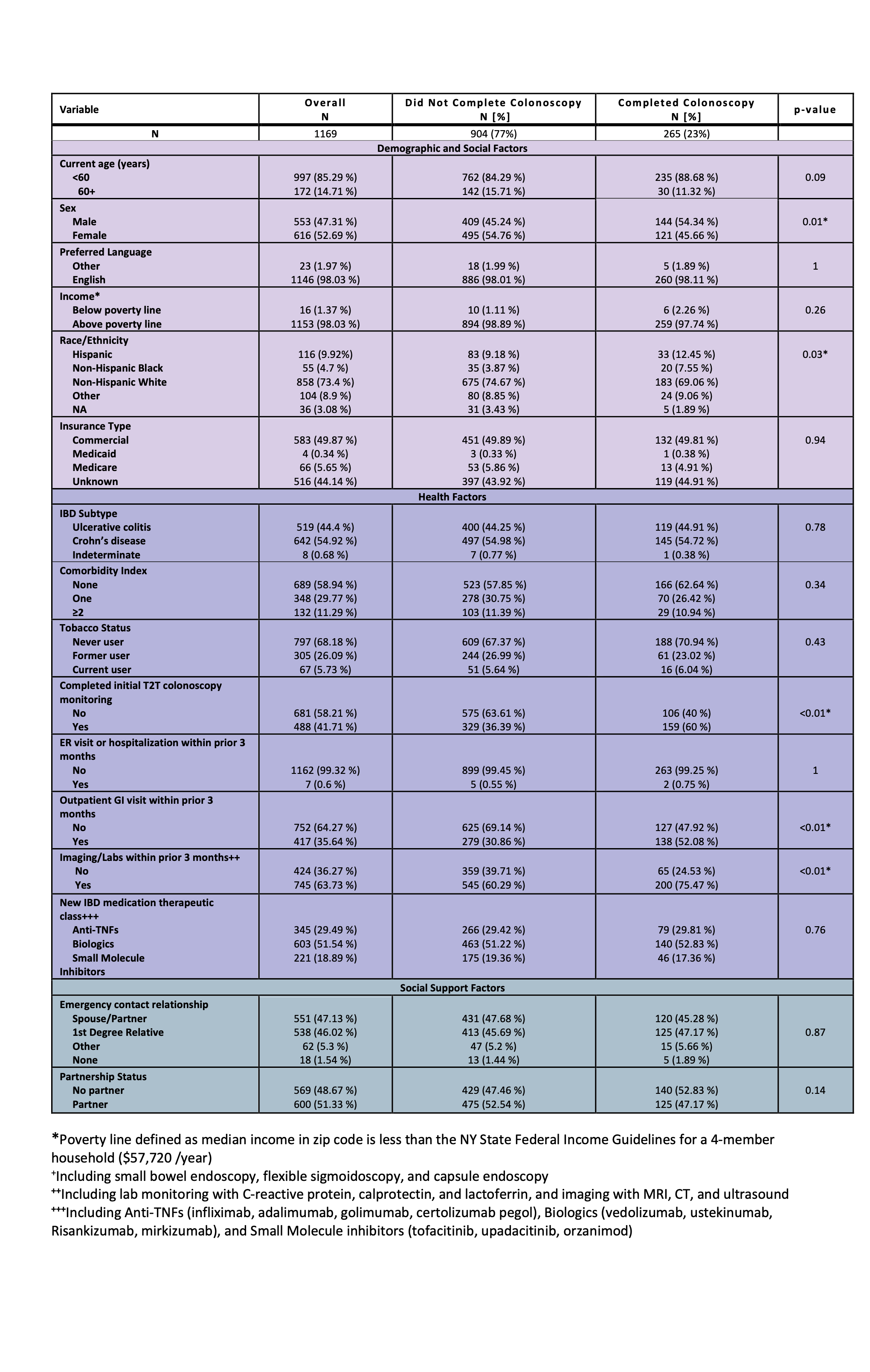
Figure: Table 1: Baseline characteristics of patients transitioning advanced IBD medication treatment by preceding objective endoscopic disease activity monitoring
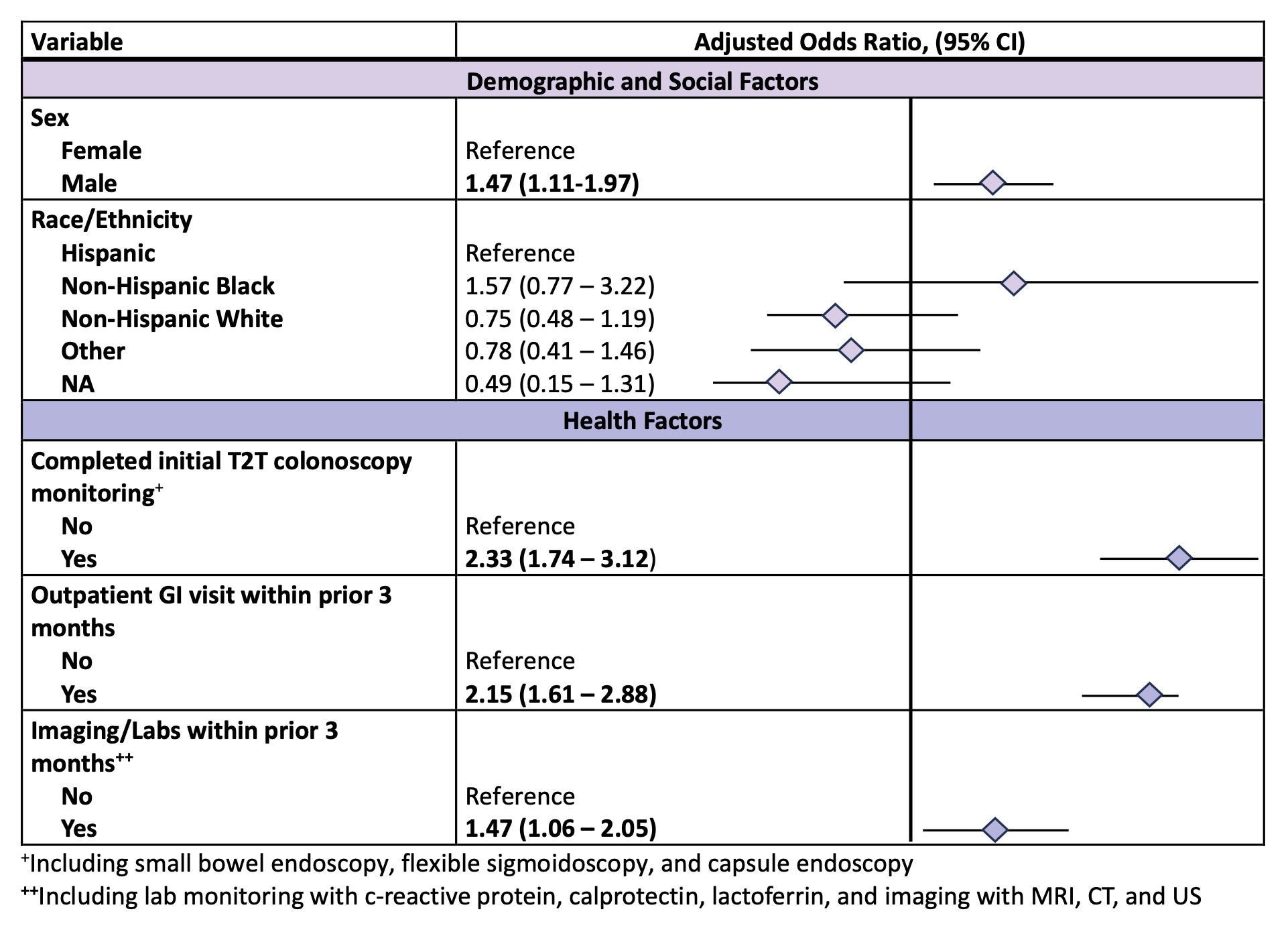
Figure: Table 2: Multivariable analysis examining predictors of objective endoscopic disease activity monitoring within 3 months prior to initiating advanced IBD medication treatment
Disclosures:
Katherine Stone indicated no relevant financial relationships.
Xucong Meng indicated no relevant financial relationships.
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Aasma Shaukat: Freenome inc – Consultant.
Adam Faye: AbbVie – Honorarium. Eli Lilly – Consultant. NIH Grant K76AG083286 – Grant/Research Support. Takeda – Honorarium. The American College of Gastroenterology – Grant/Research Support. The Crohn's and Colitis Foundation – Grant/Research Support.
Katherine L.. Stone, MD1, Xucong Meng, MPH2, Jordan Axelrad, MD, MPH3, Aasma Shaukat, MD, MPH, FACG4, Adam Faye, MD, MS1. P1123 - Uncovering Drivers of Endoscopic Monitoring Before Treatment Changes in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1NYU Langone Health, New York, NY; 2NYU Grossman School of Medicine, New York, NY; 3Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 4NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY
Introduction: Since the publication of the 2015 STRIDE guidelines, the management of inflammatory bowel disease (IBD) has increasingly adopted a treat-to-target (T2T) approach, emphasizing the achievement of endoscopic remission. Despite these recommendations, fewer than half of patients undergo follow-up endoscopy after initiating therapy. To better understand current clinical practice, we aimed to evaluate how often objective assessments (e.g., endoscopy, imaging, biomarkers) are used to guide treatment decisions, particularly among patients who have been on an advanced therapy for at least six months but subsequently undergo a change in therapy.
Methods: We performed a retrospective analysis of IBD patients (≥ 18 years) at an academic center who began a new advanced therapy between Jan 1, 2016, and May 31, 2023, and switched therapies after ≥ 6 months. Patients with prior colectomy were excluded. Demographics and clinical data were extracted from electronic medical records. Logistic regression identified factors associated with colonoscopy within 3 months before therapy change.
Results: Of 1,169 patients (519 with ulcerative colitis, 642 with Crohn’s disease, and 8 with indeterminate colitis), only 265 (23%) had colonoscopy within 3 months before switching therapies. Among the 904 patients who did not have a colonoscopy, 537 (59%) underwent lab monitoring (CRP, calprotectin, lactoferrin) and 79 (9%) had imaging (CT, MRI, ultrasound). Male sex was associated with higher odds of colonoscopy (aOR 1.47, 95% CI 1.11–1.97). Prior healthcare engagement, including a prior T2T colonoscopy (i.e., within 3-15 months of the original therapy) (aOR 2.33, 95% CI 1.74–3.12), recent gastroenterology visits (aOR 2.15, 95% CI 1.61-2.88), and non-endoscopic monitoring through labs and imaging as defined above (aOR 1.47, 95% CI 1.06-2.05), were also linked to greater likelihood of undergoing endoscopic assessment. However, the type of therapy being initiated (e.g., TNF inhibitor, other biologic, or small molecule) was not associated with differences in endoscopy use.
Discussion: Fewer than one-third of patients completed endoscopic or imaging assessments before switching advanced therapies. Prior engagement in care—especially successful T2T monitoring—was the strongest predictor of endoscopic evaluation. These findings may inform future efforts to identify and address barriers to objective monitoring, and support interventions to promote timely disease assessment before therapy changes.

Figure: Table 1: Baseline characteristics of patients transitioning advanced IBD medication treatment by preceding objective endoscopic disease activity monitoring

Figure: Table 2: Multivariable analysis examining predictors of objective endoscopic disease activity monitoring within 3 months prior to initiating advanced IBD medication treatment
Disclosures:
Katherine Stone indicated no relevant financial relationships.
Xucong Meng indicated no relevant financial relationships.
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Aasma Shaukat: Freenome inc – Consultant.
Adam Faye: AbbVie – Honorarium. Eli Lilly – Consultant. NIH Grant K76AG083286 – Grant/Research Support. Takeda – Honorarium. The American College of Gastroenterology – Grant/Research Support. The Crohn's and Colitis Foundation – Grant/Research Support.
Katherine L.. Stone, MD1, Xucong Meng, MPH2, Jordan Axelrad, MD, MPH3, Aasma Shaukat, MD, MPH, FACG4, Adam Faye, MD, MS1. P1123 - Uncovering Drivers of Endoscopic Monitoring Before Treatment Changes in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
