Sunday Poster Session
Category: IBD
P1117 - Safety of Biologic Therapies in Pregnant Women With Inflammatory Bowel Disease: A Retrospective Matched Cohort Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Mostafa Eysha, MD
Texas Tech University Health Science Center El Paso
El Paso, TX
Presenting Author(s)
Mostafa Eysha, MD1, Mohanad Elchouemi, BS2, Seyma Bayram, MD3, Bayan Khasawneh, MD4, Arsalan Asad, BS5, Mutaz Kalas, MD2, Mona A. Ali, MD6, Hend A. Elsaka, MD6, Abirami Rajendiran, MD7, Abdelrahman Yousif, MD2, Sherif E. Elhanafi, MD7
1Texas Tech University Health Science Center El Paso, El Paso, TX; 2Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center, El Paso, TX; 3The Wright Center for Graduate Medical Education, Scranton, PA; 4Houston Methodist Hospital, Houston, TX; 5John Sealy School of Medicine, University of Texas Medical Branch, Galveston, TX; 6Mansoura University, Mansoura, Ad Daqahliyah, Egypt; 7Texas Tech University Health Sciences Center, El Paso, TX
Introduction: Inflammatory Bowel Disease (IBD) frequently affects women of reproductive age, and active disease during pregnancy elevates risks of adverse maternal and fetal outcomes. Biologic therapies are essential for managing moderate to severe IBD. While continuing many biologics during pregnancy is generally supported to maintain remission, concerns about placental transfer and neonatal impacts persist, particularly for newer agents with limited long-term infant data. This study aimed to assess the association between biologic exposure during pregnancy in women with IBD and specific adverse maternal and fetal outcomes compared to non-exposed pregnant women with IBD.
Methods: This is a retrospective cohort study utilizing TriNetX, a US collaborative database, to identify pregnant women ( >18 years old) with IBD. Treatment and non-treatment groups were created based on biologic therapies use during pregnancy. Propensity score matching (1:1) was employed to minimize bias by adjusting for demographics and relevant comorbidities. Odds ratios (OR) for miscarriage, preterm delivery, suspected poor fetal growth, cesarean section rates, hypertensive disorders, and fatty liver of pregnancy were calculated to compare matched cohorts.
Results: A total of 795 patients were identified in the biologic therapies group and a total of 16,101 patients were identified in the non-biologic control group. After propensity score matching, 777 patients remained in each cohort. There was no statistical difference in maternal and fetal outcomes between the two groups. Specifically, these included miscarriage (OR 1.0, 95% CI 0.51-1.97), preterm delivery (OR 0.69, 95% CI 0.43-1.11), suspected poor fetal growth (OR 0.85, 95% CI 0.57-1.26), cesarean section (OR 1.03, 95% CI 0.74-1.42), hypertensive disorders of pregnancy (OR 0.81, 95% CI 0.55-1.19), and fatty liver of pregnancy (OR 1.0, 95% CI 0.41-2.42)(Table 1).
Discussion: In this retrospective cohort study, biologic therapies in pregnant patients with IBD appear to be safe, showing no increased risk of miscarriage, preterm delivery, suspected fetal growth restriction, hypertensive disorders of pregnancy, or cesarean delivery compared to the control group. These findings support the continued evaluation of biologics as a potentially safe option during pregnancy in IBD patients.
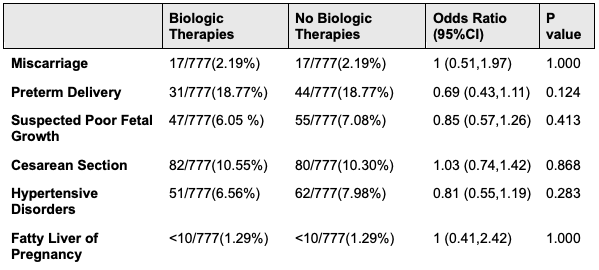
Figure: Table 1. Adverse outcomes in pregnant women with IBD receiving biologic therapies versus those not receiving biologic therapies
Disclosures:
Mostafa Eysha indicated no relevant financial relationships.
Mohanad Elchouemi indicated no relevant financial relationships.
Seyma Bayram indicated no relevant financial relationships.
Bayan Khasawneh indicated no relevant financial relationships.
Arsalan Asad indicated no relevant financial relationships.
Mutaz Kalas indicated no relevant financial relationships.
Mona Ali indicated no relevant financial relationships.
Hend Elsaka indicated no relevant financial relationships.
Abirami Rajendiran indicated no relevant financial relationships.
Abdelrahman Yousif indicated no relevant financial relationships.
Sherif Elhanafi indicated no relevant financial relationships.
Mostafa Eysha, MD1, Mohanad Elchouemi, BS2, Seyma Bayram, MD3, Bayan Khasawneh, MD4, Arsalan Asad, BS5, Mutaz Kalas, MD2, Mona A. Ali, MD6, Hend A. Elsaka, MD6, Abirami Rajendiran, MD7, Abdelrahman Yousif, MD2, Sherif E. Elhanafi, MD7. P1117 - Safety of Biologic Therapies in Pregnant Women With Inflammatory Bowel Disease: A Retrospective Matched Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Texas Tech University Health Science Center El Paso, El Paso, TX; 2Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center, El Paso, TX; 3The Wright Center for Graduate Medical Education, Scranton, PA; 4Houston Methodist Hospital, Houston, TX; 5John Sealy School of Medicine, University of Texas Medical Branch, Galveston, TX; 6Mansoura University, Mansoura, Ad Daqahliyah, Egypt; 7Texas Tech University Health Sciences Center, El Paso, TX
Introduction: Inflammatory Bowel Disease (IBD) frequently affects women of reproductive age, and active disease during pregnancy elevates risks of adverse maternal and fetal outcomes. Biologic therapies are essential for managing moderate to severe IBD. While continuing many biologics during pregnancy is generally supported to maintain remission, concerns about placental transfer and neonatal impacts persist, particularly for newer agents with limited long-term infant data. This study aimed to assess the association between biologic exposure during pregnancy in women with IBD and specific adverse maternal and fetal outcomes compared to non-exposed pregnant women with IBD.
Methods: This is a retrospective cohort study utilizing TriNetX, a US collaborative database, to identify pregnant women ( >18 years old) with IBD. Treatment and non-treatment groups were created based on biologic therapies use during pregnancy. Propensity score matching (1:1) was employed to minimize bias by adjusting for demographics and relevant comorbidities. Odds ratios (OR) for miscarriage, preterm delivery, suspected poor fetal growth, cesarean section rates, hypertensive disorders, and fatty liver of pregnancy were calculated to compare matched cohorts.
Results: A total of 795 patients were identified in the biologic therapies group and a total of 16,101 patients were identified in the non-biologic control group. After propensity score matching, 777 patients remained in each cohort. There was no statistical difference in maternal and fetal outcomes between the two groups. Specifically, these included miscarriage (OR 1.0, 95% CI 0.51-1.97), preterm delivery (OR 0.69, 95% CI 0.43-1.11), suspected poor fetal growth (OR 0.85, 95% CI 0.57-1.26), cesarean section (OR 1.03, 95% CI 0.74-1.42), hypertensive disorders of pregnancy (OR 0.81, 95% CI 0.55-1.19), and fatty liver of pregnancy (OR 1.0, 95% CI 0.41-2.42)(Table 1).
Discussion: In this retrospective cohort study, biologic therapies in pregnant patients with IBD appear to be safe, showing no increased risk of miscarriage, preterm delivery, suspected fetal growth restriction, hypertensive disorders of pregnancy, or cesarean delivery compared to the control group. These findings support the continued evaluation of biologics as a potentially safe option during pregnancy in IBD patients.

Figure: Table 1. Adverse outcomes in pregnant women with IBD receiving biologic therapies versus those not receiving biologic therapies
Disclosures:
Mostafa Eysha indicated no relevant financial relationships.
Mohanad Elchouemi indicated no relevant financial relationships.
Seyma Bayram indicated no relevant financial relationships.
Bayan Khasawneh indicated no relevant financial relationships.
Arsalan Asad indicated no relevant financial relationships.
Mutaz Kalas indicated no relevant financial relationships.
Mona Ali indicated no relevant financial relationships.
Hend Elsaka indicated no relevant financial relationships.
Abirami Rajendiran indicated no relevant financial relationships.
Abdelrahman Yousif indicated no relevant financial relationships.
Sherif Elhanafi indicated no relevant financial relationships.
Mostafa Eysha, MD1, Mohanad Elchouemi, BS2, Seyma Bayram, MD3, Bayan Khasawneh, MD4, Arsalan Asad, BS5, Mutaz Kalas, MD2, Mona A. Ali, MD6, Hend A. Elsaka, MD6, Abirami Rajendiran, MD7, Abdelrahman Yousif, MD2, Sherif E. Elhanafi, MD7. P1117 - Safety of Biologic Therapies in Pregnant Women With Inflammatory Bowel Disease: A Retrospective Matched Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
