Sunday Poster Session
Category: IBD
P1113 - Recent Recall as an Alternative to Daily Study Participant Diaries in Ulcerative Colitis Trials: Evidence From a Phase 3 Clinical Trial
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Raj Devarajan, MD
Shivkar LLC
Acton, MA
Presenting Author(s)
Raj Devarajan, MD1, Philip Lavin, PhD2, Mark Ensign, 3, Jennifer Davagian, 3
1Shivkar LLC, Acton, MA; 2Lavin Consulting, LLC, Framingham, MA; 3Cristcot, Concord, MA
Introduction: Study participant diaries and direct recall are commonly used to collect disease symptoms in clinical research. Daily diaries can be burdensome, exemplified by poor adherence with daily reporting, and are subject to recall bias. The objective of this analysis was to explore the relationship between the use of a daily diary versus recent recall of disease symptom subscores of the Modified Mayo Score (MMS) components within an ulcerative colitis (UC) clinical trial.
Methods: CESSA (NCT04469686) was a double-blind, placebo-controlled, phase 3 trial evaluating a novel, investigational 90 mg hydrocortisone acetate (HCA) suppository formulation, administered once or twice daily for 28 days using a proprietary FDA-cleared applicator, in adults with endoscopically confirmed active moderate-to-severe UC of the rectum. MMS components of stool frequency and rectal bleeding were evaluated at baseline, Day 15, and Day 29 and compared with corresponding data from daily study participant diaries (averaged) and study participant recall of the prior 3 days. Pearson product-moment methodology was used to calculate correlation coefficients for MMS subscores and diary- or recent recall-based reporting at each timepoint for study participants with complete data.
Results: For both pooled data and individual study arms, study participant-reported data were consistent with MMS subscores; however, recent recall data more closely matched subscores and diary data were associated with an overall under-reporting of symptoms by 10% (Table). Pearson correlation coefficients between recent recall and diary data for stool frequency and rectal bleeding ranged from 0.61 to 0.85 across time points, and accounted for only 37%–72% of the observed variance from MMS subscores. No trends in degree of correlation were evident across treatment arms.
Discussion: Recent recall is a recognized standard used in many study participant-reported outcome tools. This analysis revealed limited correlation of data from daily study participant diaries and recent recall, with evidence of more mild MMS component reporting for diary data and unexplained variance between diary and recent recall data. Given the known limitations of diary data and gradual daily change in symptoms of UC, recent recall of MMS components should be considered as a standard in future UC studies.
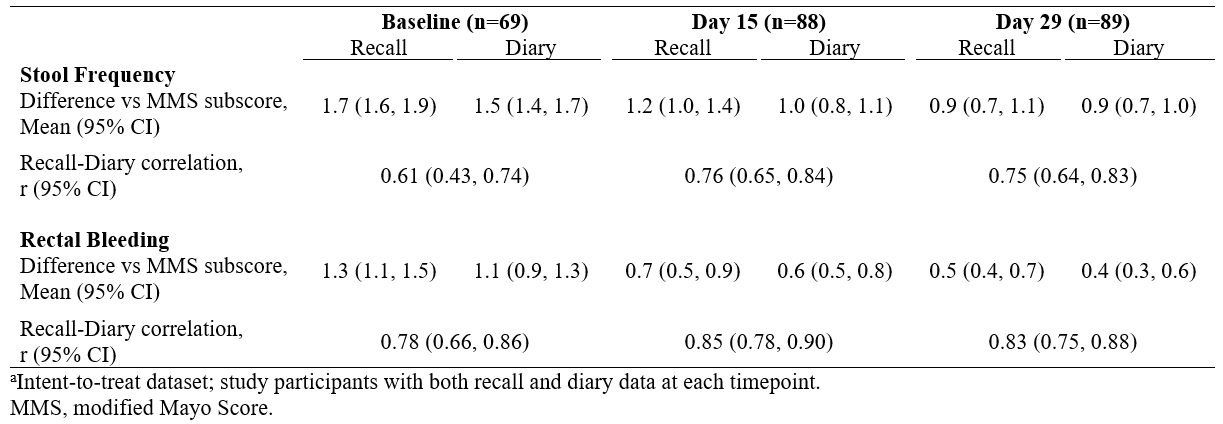
Figure: Comparison Study Participant-Reported Modified Mayo Score Components in Adults With Active Ulcerative Colitis of the Rectum[a]
Disclosures:
Raj Devarajan: Cristcot – Consultant.
Philip Lavin: Cristcot – Consultant.
Mark Ensign: Cristcot – Employee.
Jennifer Davagian: Cristcot – Employee, Equity.
Raj Devarajan, MD1, Philip Lavin, PhD2, Mark Ensign, 3, Jennifer Davagian, 3. P1113 - Recent Recall as an Alternative to Daily Study Participant Diaries in Ulcerative Colitis Trials: Evidence From a Phase 3 Clinical Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Shivkar LLC, Acton, MA; 2Lavin Consulting, LLC, Framingham, MA; 3Cristcot, Concord, MA
Introduction: Study participant diaries and direct recall are commonly used to collect disease symptoms in clinical research. Daily diaries can be burdensome, exemplified by poor adherence with daily reporting, and are subject to recall bias. The objective of this analysis was to explore the relationship between the use of a daily diary versus recent recall of disease symptom subscores of the Modified Mayo Score (MMS) components within an ulcerative colitis (UC) clinical trial.
Methods: CESSA (NCT04469686) was a double-blind, placebo-controlled, phase 3 trial evaluating a novel, investigational 90 mg hydrocortisone acetate (HCA) suppository formulation, administered once or twice daily for 28 days using a proprietary FDA-cleared applicator, in adults with endoscopically confirmed active moderate-to-severe UC of the rectum. MMS components of stool frequency and rectal bleeding were evaluated at baseline, Day 15, and Day 29 and compared with corresponding data from daily study participant diaries (averaged) and study participant recall of the prior 3 days. Pearson product-moment methodology was used to calculate correlation coefficients for MMS subscores and diary- or recent recall-based reporting at each timepoint for study participants with complete data.
Results: For both pooled data and individual study arms, study participant-reported data were consistent with MMS subscores; however, recent recall data more closely matched subscores and diary data were associated with an overall under-reporting of symptoms by 10% (Table). Pearson correlation coefficients between recent recall and diary data for stool frequency and rectal bleeding ranged from 0.61 to 0.85 across time points, and accounted for only 37%–72% of the observed variance from MMS subscores. No trends in degree of correlation were evident across treatment arms.
Discussion: Recent recall is a recognized standard used in many study participant-reported outcome tools. This analysis revealed limited correlation of data from daily study participant diaries and recent recall, with evidence of more mild MMS component reporting for diary data and unexplained variance between diary and recent recall data. Given the known limitations of diary data and gradual daily change in symptoms of UC, recent recall of MMS components should be considered as a standard in future UC studies.

Figure: Comparison Study Participant-Reported Modified Mayo Score Components in Adults With Active Ulcerative Colitis of the Rectum[a]
Disclosures:
Raj Devarajan: Cristcot – Consultant.
Philip Lavin: Cristcot – Consultant.
Mark Ensign: Cristcot – Employee.
Jennifer Davagian: Cristcot – Employee, Equity.
Raj Devarajan, MD1, Philip Lavin, PhD2, Mark Ensign, 3, Jennifer Davagian, 3. P1113 - Recent Recall as an Alternative to Daily Study Participant Diaries in Ulcerative Colitis Trials: Evidence From a Phase 3 Clinical Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
