Sunday Poster Session
Category: IBD
P1112 - Efficacy and Safety of a Novel Investigational Hydrocortisone Acetate Suppository Formulation and Optimized Applicator for Treatment of Active Ulcerative Colitis: Results of the Phase 3 CESSA Trial
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Raj Devarajan, MD
Shivkar LLC
Acton, MA
Presenting Author(s)
Award: ACG Presidential Poster Award
Raj Devarajan, MD1, Philip Lavin, PhD2, Mark Ensign, 3, Jennifer Davagian, 3
1Shivkar LLC, Acton, MA; 2Lavin Consulting, LLC, Framingham, MA; 3Cristcot, Concord, MA
Introduction: Rectally administered corticosteroids (CS) are used as acute therapy for active ulcerative colitis (UC). FDA-approved, liquid and foam CS treat the colon proximal to the rectum, and are associated with suboptimal efficacy and negative impact on quality of life due to administration difficulty, medication leakage, and discomfort. This phase 3 trial evaluated the efficacy and safety of a novel, investigational 90 mg hydrocortisone acetate (HCA) suppository delivered using a proprietary applicator for optimized CS treatment.
Methods: CESSA (NCT04469686) was a randomized, double-blind, placebo-controlled, multinational trial conducted in adults with confirmed active moderate-to-severe UC of the rectum (endoscopic score, 2–3; and total Modified Mayo Score [MMS], 4–8). Study participants were randomized 1:1:1 to receive investigational suppositories of HCA 90 mg once daily (qd; with nightly placebo), HCA 90 mg twice daily (bid), or placebo bid for 28 days, followed by a 10-day taper (daily to every other day). Use of select maintenance therapies, but not CS, was permitted. The primary endpoint was clinical remission at Day 29, defined as a total MMS of 0–2, including zero bleeding, reduction in stool frequency, and mucosal healing (Table 1). Data were analyzed in the overall intent-to-treat (ITT) set; a subgroup analysis for response at Day 15 was performed for study participants who achieved clinical remission at Day 29.
Results: Among the 200 study participants enrolled, 65% remained on maintenance therapy for UC and 35% received no maintenance therapy. Rectal bleeding (score, 1–2) was present in 84% of study participants at baseline. Clinical remission was achieved by significantly more study participants in the HCA qd and HCA bid arms than in the placebo arm (ITT [n=199]; 21% and 16% vs 1.5%, respectively; Table 2). Clinical responses were evident by Day 15, and among study participants with clinical remission at Day 29 (n=26), nearly all met criteria for MMS component responses at Day 15 (stool frequency, 96%; rectal bleeding, 100%; Table 2). Adverse events (AEs) were generally mild and consistent with the known safety profile of rectal CS, and no serious AEs were reported. Dosing adherence was high (~90%) across study arms.
Discussion: In study participants with active UC of the rectum, rapid-release HCA 90 mg suppositories showed rapid onset of effect and statistical significance over placebo in inducing and confirming clinical remission and was well tolerated.
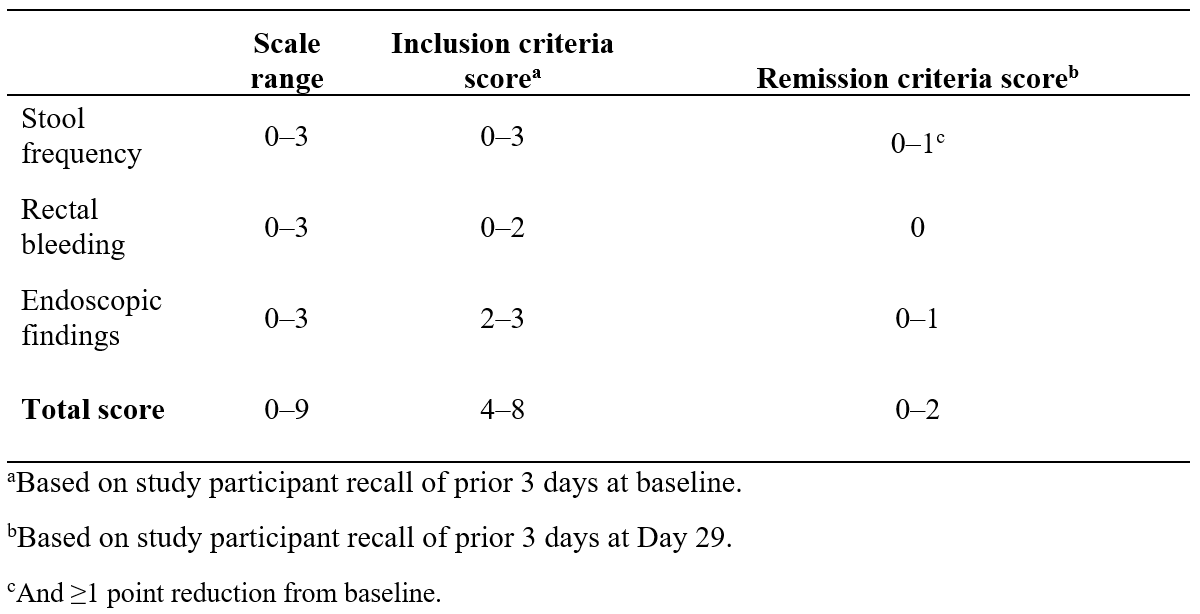
Figure: Table 1. Primary Endpoint Definitions (Modified Mayo Score)
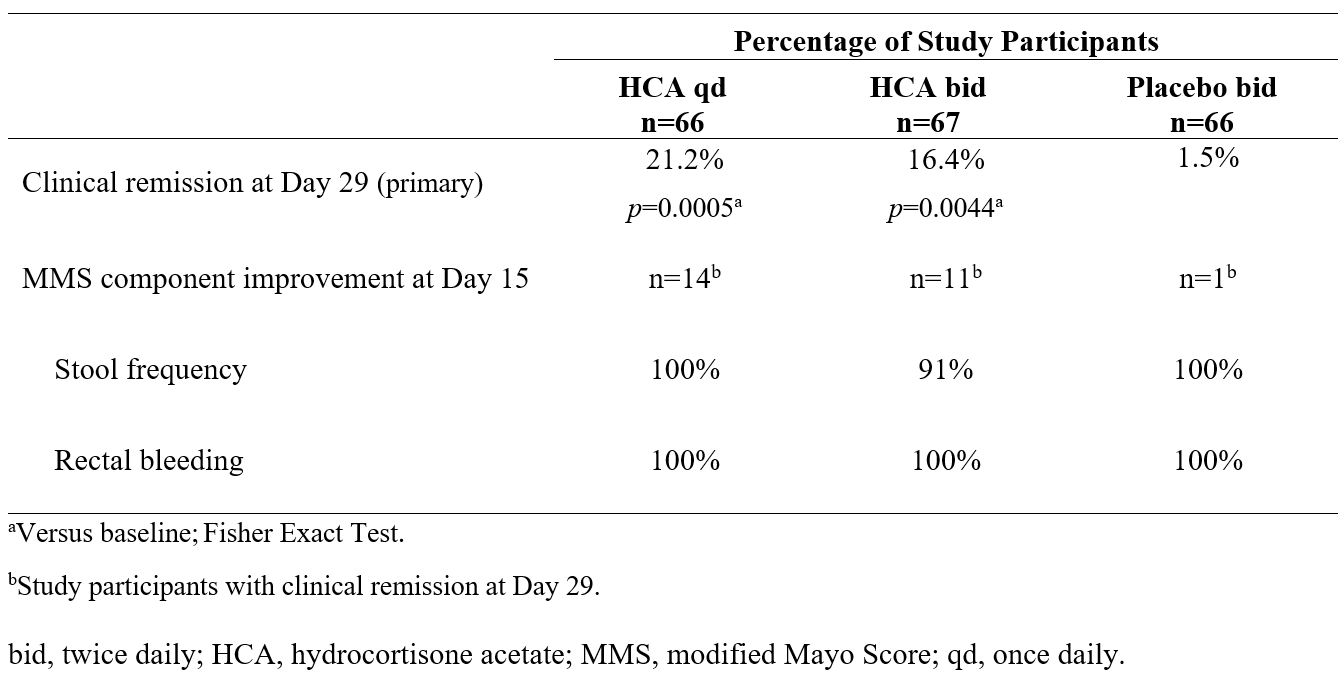
Figure: Table 2. Efficacy Outcomes Through Day 29
Disclosures:
Raj Devarajan: Cristcot – Consultant.
Philip Lavin: Cristcot – Consultant.
Mark Ensign: Cristcot – Employee.
Jennifer Davagian: Cristcot – Employee, Equity.
Raj Devarajan, MD1, Philip Lavin, PhD2, Mark Ensign, 3, Jennifer Davagian, 3. P1112 - Efficacy and Safety of a Novel Investigational Hydrocortisone Acetate Suppository Formulation and Optimized Applicator for Treatment of Active Ulcerative Colitis: Results of the Phase 3 CESSA Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Raj Devarajan, MD1, Philip Lavin, PhD2, Mark Ensign, 3, Jennifer Davagian, 3
1Shivkar LLC, Acton, MA; 2Lavin Consulting, LLC, Framingham, MA; 3Cristcot, Concord, MA
Introduction: Rectally administered corticosteroids (CS) are used as acute therapy for active ulcerative colitis (UC). FDA-approved, liquid and foam CS treat the colon proximal to the rectum, and are associated with suboptimal efficacy and negative impact on quality of life due to administration difficulty, medication leakage, and discomfort. This phase 3 trial evaluated the efficacy and safety of a novel, investigational 90 mg hydrocortisone acetate (HCA) suppository delivered using a proprietary applicator for optimized CS treatment.
Methods: CESSA (NCT04469686) was a randomized, double-blind, placebo-controlled, multinational trial conducted in adults with confirmed active moderate-to-severe UC of the rectum (endoscopic score, 2–3; and total Modified Mayo Score [MMS], 4–8). Study participants were randomized 1:1:1 to receive investigational suppositories of HCA 90 mg once daily (qd; with nightly placebo), HCA 90 mg twice daily (bid), or placebo bid for 28 days, followed by a 10-day taper (daily to every other day). Use of select maintenance therapies, but not CS, was permitted. The primary endpoint was clinical remission at Day 29, defined as a total MMS of 0–2, including zero bleeding, reduction in stool frequency, and mucosal healing (Table 1). Data were analyzed in the overall intent-to-treat (ITT) set; a subgroup analysis for response at Day 15 was performed for study participants who achieved clinical remission at Day 29.
Results: Among the 200 study participants enrolled, 65% remained on maintenance therapy for UC and 35% received no maintenance therapy. Rectal bleeding (score, 1–2) was present in 84% of study participants at baseline. Clinical remission was achieved by significantly more study participants in the HCA qd and HCA bid arms than in the placebo arm (ITT [n=199]; 21% and 16% vs 1.5%, respectively; Table 2). Clinical responses were evident by Day 15, and among study participants with clinical remission at Day 29 (n=26), nearly all met criteria for MMS component responses at Day 15 (stool frequency, 96%; rectal bleeding, 100%; Table 2). Adverse events (AEs) were generally mild and consistent with the known safety profile of rectal CS, and no serious AEs were reported. Dosing adherence was high (~90%) across study arms.
Discussion: In study participants with active UC of the rectum, rapid-release HCA 90 mg suppositories showed rapid onset of effect and statistical significance over placebo in inducing and confirming clinical remission and was well tolerated.

Figure: Table 1. Primary Endpoint Definitions (Modified Mayo Score)

Figure: Table 2. Efficacy Outcomes Through Day 29
Disclosures:
Raj Devarajan: Cristcot – Consultant.
Philip Lavin: Cristcot – Consultant.
Mark Ensign: Cristcot – Employee.
Jennifer Davagian: Cristcot – Employee, Equity.
Raj Devarajan, MD1, Philip Lavin, PhD2, Mark Ensign, 3, Jennifer Davagian, 3. P1112 - Efficacy and Safety of a Novel Investigational Hydrocortisone Acetate Suppository Formulation and Optimized Applicator for Treatment of Active Ulcerative Colitis: Results of the Phase 3 CESSA Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

