Sunday Poster Session
Category: IBD
P1078 - Comparative Effectiveness of Ustekinumab and Ozanimod in Anti-TNF Naïve Patients With Ulcerative Colitis: A Real-World Matched Cohort Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Mohamed Eldesouki, MD
Saint Michael's Medical Center, New York Medical College
Newark, NJ
Presenting Author(s)
Mohamed Eldesouki, MD1, Khaled Elfert, MBChB, MRCP2, Ahmed Ibrahim, MD3, Ahmed Salem, MD4, Ismail Althunibat, MD5, Murad Qirem, MD6, Yahya Alhalalmeh, MD5, Hazem Abosheaishaa, MD7, Jennifer Hadam-Veverka, MD2
1Saint Michael's Medical Center, New York Medical College, Newark, NJ; 2West Virginia University School of Medicine, Morgantown, WV; 3Medical University of South Carolina, Charleston, SC; 4Maimonides Medical Center, Brooklyn, NY; 5New York Medical College - Saint Michael's Medical Center, Newark, NJ; 6New York Medical College - Saint Michael's Medical Center, West Orange, NJ; 7Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY
Introduction: Ustekinumab and ozanimod are approved for the management of moderate-to-severe ulcerative colitis (UC). However, real-world data comparing their effectiveness in anti-TNF naïve patients remains scarce. This study aimed to compare the 1-year clinical outcomes of ustekinumab versus ozanimod in anti-TNF naïve UC patients using a large real-world database.
Methods: We performed a retrospective cohort study using the TriNetX U.S. Collaborative Research Network to compare the 1-year outcomes of anti-TNF naïve adult patients with UC who initiated ustekinumab or ozanimod between January 2021 and April 2024. Patients were matched 1:1 using propensity score matching (PSM) for demographics, comorbidities, and baseline laboratory parameters. The primary outcome was corticosteroid use at 12 months. Secondary outcomes included rates of surgery, inpatient hospitalizations, emergency department (ED) visits, and subsequent anti-TNF initiation.
Results: A total of 1,926 patients with ulcerative colitis were identified, including 307 who initiated ozanimod (2021-2024). After matching, 306 patients were included in each treatment arm. Baseline characteristics were balanced between groups (mean age: 42 ± 17 vs. 42 ± 16 years; female: 37% vs. 40%; p > 0.05 across all clinical variables; Table). At 1 year, corticosteroid use was similar between ustekinumab and ozanimod (39.5% vs. 40.9%; aOR: 1.06, 95% CI: 0.81–1.53; p = 0.70; Figure). There were no significant differences in surgical intervention (3.6% vs. 3.3%; aOR: 1.02, 95% CI: 0.42–2.30; p = 0.60; Figure), inpatient hospitalizations (3.2% vs. 3.3%; aOR: 1.06, 95% CI: 0.42–2.30; p = 0.90; Figure), or emergency department (ED) visits (10.8% vs. 10.5%; aOR: 1.03, 95% CI: 0.62–1.73; p = 0.80; Figure). A trend toward reduced anti-TNF initiation was observed in the ustekinumab group (3.3% vs. 6.2%; aOR: 1.96, 95% CI: 0.89–4.29; p = 0.10; Figure).
Discussion: Our real-world study showed that among anti-TNF naïve UC patients, ustekinumab and ozanimod demonstrated comparable 1-year effectiveness with similar corticosteroid use and healthcare utilization outcomes. Further prospective studies are needed to understand long-term outcomes.
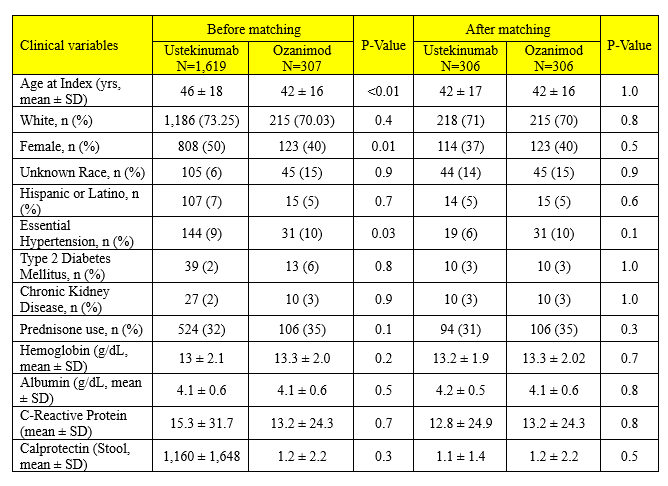
Figure: Table. Clinical variables before and after propensity score matching.
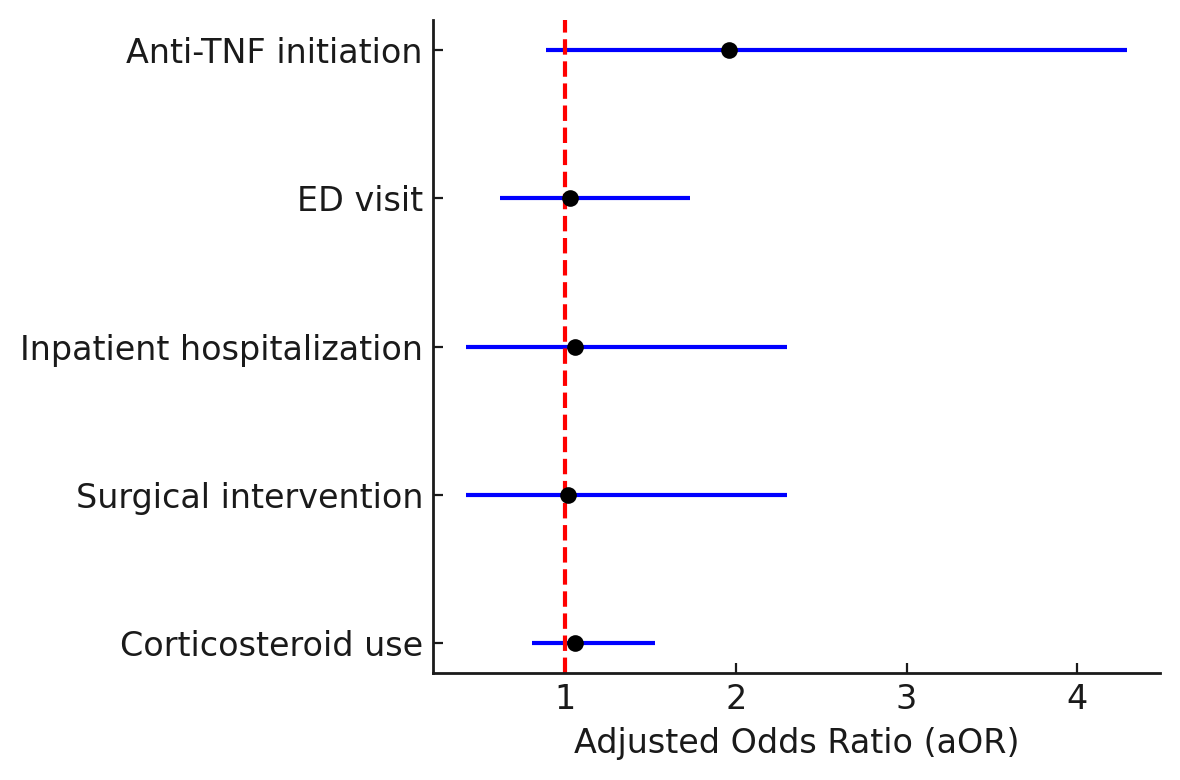
Figure: Figure. Forest plot depicting the 1-year clinical outcomes in anti-TNF naïve ulcerative colitis patients treated with ustekinumab versus ozanimod.
Disclosures:
Mohamed Eldesouki indicated no relevant financial relationships.
Khaled Elfert indicated no relevant financial relationships.
Ahmed Ibrahim indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Ismail Althunibat indicated no relevant financial relationships.
Murad Qirem indicated no relevant financial relationships.
Yahya Alhalalmeh indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Jennifer Hadam-Veverka: Abbvie – Speakers Bureau. Johnson & Johnson – Speakers Bureau. Takeda – Speakers Bureau.
Mohamed Eldesouki, MD1, Khaled Elfert, MBChB, MRCP2, Ahmed Ibrahim, MD3, Ahmed Salem, MD4, Ismail Althunibat, MD5, Murad Qirem, MD6, Yahya Alhalalmeh, MD5, Hazem Abosheaishaa, MD7, Jennifer Hadam-Veverka, MD2. P1078 - Comparative Effectiveness of Ustekinumab and Ozanimod in Anti-TNF Naïve Patients With Ulcerative Colitis: A Real-World Matched Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Saint Michael's Medical Center, New York Medical College, Newark, NJ; 2West Virginia University School of Medicine, Morgantown, WV; 3Medical University of South Carolina, Charleston, SC; 4Maimonides Medical Center, Brooklyn, NY; 5New York Medical College - Saint Michael's Medical Center, Newark, NJ; 6New York Medical College - Saint Michael's Medical Center, West Orange, NJ; 7Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY
Introduction: Ustekinumab and ozanimod are approved for the management of moderate-to-severe ulcerative colitis (UC). However, real-world data comparing their effectiveness in anti-TNF naïve patients remains scarce. This study aimed to compare the 1-year clinical outcomes of ustekinumab versus ozanimod in anti-TNF naïve UC patients using a large real-world database.
Methods: We performed a retrospective cohort study using the TriNetX U.S. Collaborative Research Network to compare the 1-year outcomes of anti-TNF naïve adult patients with UC who initiated ustekinumab or ozanimod between January 2021 and April 2024. Patients were matched 1:1 using propensity score matching (PSM) for demographics, comorbidities, and baseline laboratory parameters. The primary outcome was corticosteroid use at 12 months. Secondary outcomes included rates of surgery, inpatient hospitalizations, emergency department (ED) visits, and subsequent anti-TNF initiation.
Results: A total of 1,926 patients with ulcerative colitis were identified, including 307 who initiated ozanimod (2021-2024). After matching, 306 patients were included in each treatment arm. Baseline characteristics were balanced between groups (mean age: 42 ± 17 vs. 42 ± 16 years; female: 37% vs. 40%; p > 0.05 across all clinical variables; Table). At 1 year, corticosteroid use was similar between ustekinumab and ozanimod (39.5% vs. 40.9%; aOR: 1.06, 95% CI: 0.81–1.53; p = 0.70; Figure). There were no significant differences in surgical intervention (3.6% vs. 3.3%; aOR: 1.02, 95% CI: 0.42–2.30; p = 0.60; Figure), inpatient hospitalizations (3.2% vs. 3.3%; aOR: 1.06, 95% CI: 0.42–2.30; p = 0.90; Figure), or emergency department (ED) visits (10.8% vs. 10.5%; aOR: 1.03, 95% CI: 0.62–1.73; p = 0.80; Figure). A trend toward reduced anti-TNF initiation was observed in the ustekinumab group (3.3% vs. 6.2%; aOR: 1.96, 95% CI: 0.89–4.29; p = 0.10; Figure).
Discussion: Our real-world study showed that among anti-TNF naïve UC patients, ustekinumab and ozanimod demonstrated comparable 1-year effectiveness with similar corticosteroid use and healthcare utilization outcomes. Further prospective studies are needed to understand long-term outcomes.

Figure: Table. Clinical variables before and after propensity score matching.

Figure: Figure. Forest plot depicting the 1-year clinical outcomes in anti-TNF naïve ulcerative colitis patients treated with ustekinumab versus ozanimod.
Disclosures:
Mohamed Eldesouki indicated no relevant financial relationships.
Khaled Elfert indicated no relevant financial relationships.
Ahmed Ibrahim indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Ismail Althunibat indicated no relevant financial relationships.
Murad Qirem indicated no relevant financial relationships.
Yahya Alhalalmeh indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Jennifer Hadam-Veverka: Abbvie – Speakers Bureau. Johnson & Johnson – Speakers Bureau. Takeda – Speakers Bureau.
Mohamed Eldesouki, MD1, Khaled Elfert, MBChB, MRCP2, Ahmed Ibrahim, MD3, Ahmed Salem, MD4, Ismail Althunibat, MD5, Murad Qirem, MD6, Yahya Alhalalmeh, MD5, Hazem Abosheaishaa, MD7, Jennifer Hadam-Veverka, MD2. P1078 - Comparative Effectiveness of Ustekinumab and Ozanimod in Anti-TNF Naïve Patients With Ulcerative Colitis: A Real-World Matched Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
