Sunday Poster Session
Category: IBD
P1071 - Efficacy of Etrasimod as First-Line Advanced Treatment Following Failure of Aminosalicylates Only: Data From the ELEVATE UC 52 Phase 3 Clinical Trial
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

David T. Rubin, MD
University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA
Chicago, IL
Presenting Author(s)
David T. Rubin, MD1, Charlie W. Lees, PhD2, Filip Baert, MD3, Maria Kudela, PhD4, Abhishek Bhattacharjee, PhD5, Krisztina Lazin, MD6, Martina Goetsch, MD6, Arcangelo M. Abbatemarco, MD7, Karolina Wosik, MSc, PhD8, John K. Marshall, MSc, MD, BA9
1University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 2Institute of Genetics & Molecular Medicine, University of Edinburgh, Edinburgh, UK; Edinburgh IBD Unit, Western General Hospital, NHS Lothian, Edinburgh, UK, Edinburgh, Scotland, United Kingdom; 3Department of Gastroenterology, AZ Delta, Roeselare, Belgium, Roeselare, West-Vlaanderen, Belgium; 4Pfizer Inc, Cambridge, MA, USA, Cambridge, MA; 5Pfizer Healthcare India Private Ltd, Chennai, India, Chennai, Tamil Nadu, India; 6Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 7Pfizer Inc, New York, NY, USA, New York, NY; 8Pfizer Canada, Kirkland, PQ, Canada; 9Division of Gastroenterology, Department of Medicine, Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, ON, Canada, Hamilton, ON, Canada
Introduction: Etrasimod is an oral, once-daily, selective sphingosine 1‑phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). In patients with moderately to severely active UC, treatment guidelines recommend early use of advanced therapies rather than gradual step up after failure of 5-aminosalicylate (5-ASA).1
Methods: This prespecified subgroup analysis assessed the efficacy of etrasimod vs placebo in patients in ELEVATE UC 52 (NCT03945188) with, and without, prior oral 5-ASA failure only (defined as inadequate response, loss of response or intolerance to previous treatment with oral 5-ASA but not to any other previous UC medication). Efficacy endpoints assessed at Weeks 12 and 52 included clinical remission, endoscopic improvement, symptomatic remission and composite histological endpoints.
Results: In total, 45 and 29 patients receiving etrasimod and placebo, respectively, had prior 5-ASA failure only and 244 and 115 patients did not. At Weeks 12 and 52, among those who had failed prior 5-ASA only, significantly more patients receiving etrasimod vs placebo achieved clinical remission (Week 12, % difference [Δ]: 38.3%, p < 0.001; Week 52, Δ: 45.0%, p < 0.001), endoscopic improvement (Week 12, Δ: 54.7%, p < 0.001; Week 52, Δ: 47.8%, p < 0.001), symptomatic remission (Week 12, Δ: 38.6%, p < 0.001; Week 52, Δ: 45.0%, p < 0.001), and endoscopic improvement-histologic remission (Week 12, Δ: 35.6%, p < 0.001; Week 52, Δ: 31.1%, p < 0.001; Table). Significantly more patients receiving etrasimod vs placebo in this subgroup achieved endoscopic normalization-histologic remission (Week 12, Δ: 19.9%, p</em> = 0.002; Week 52, Δ: 24.3%, p < 0.001) and disease clearance (Week 12, Δ: 15.6%, p</em> = 0.008; Week 52, Δ: 27.8%, p < 0.001). Among those who had failed other therapies, significantly more patients receiving etrasimod vs placebo also achieved all endpoints at Weeks 12 and 52 (p < 0.01).
Discussion: Etrasimod demonstrated robust efficacy as first-line advanced therapy in patients who had failed oral 5-ASA only, across all evaluated endpoints including stringent composite histological endpoints, consistent with the overall ELEVATE UC population. Etrasimod shows efficacy when used early in the UC treatment algorithm.
Reference:
1. Singh S et al. Gastroenterology 2024; 167: 1307–1343.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.
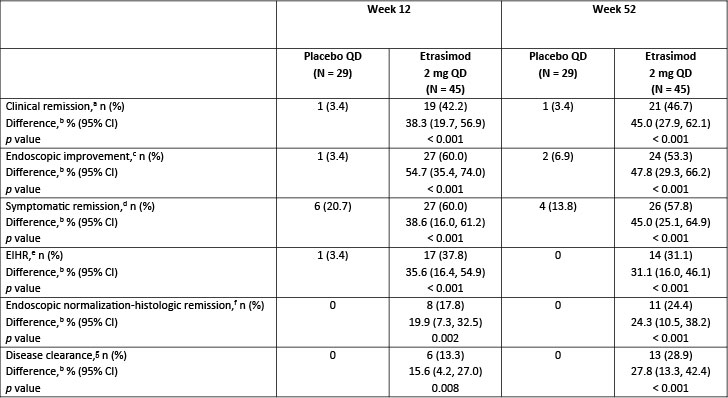
Figure: Table. Key efficacy endpoints achieved by patients with prior oral 5-ASA treatment failure only in ELEVATE UC 52.
[a]Clinical remission: SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline), RBS = 0, and ES ≤ 1 (excluding friability).
[b]Difference (95% CI) and 2-sided p value are based on the Cochran–Mantel–Haenszel method adjusting to reported randomization stratification of (1) naïve to biologic or JAK inhibitor therapy at study entry, (2) baseline corticosteroid use, and (3) baseline disease activity (MMS 4–6 or 7–9).
[c]Endoscopic improvement: ES ≤ 1.
[d]Symptomatic remission: SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline) and RBS = 0.
[e]EIHR: ES ≤ 1 with histologic remission (Geboes Index score < 2.0).
[f]Endoscopic normalization-histologic remission: Geboes Index score < 2.0 and ES = 0.
[g]Disease clearance: NHI = 0 and ES = 0 and RBS = 0 and SFS = 0 or 1 (if 1, must have ≥ 1-point decrease from baseline).
5-ASA, 5-aminosalicylate; CI, confidence interval; EIHR, endoscopic improvement-histologic remission; ES, endoscopic subscore; JAK, Janus kinase; MMS, modified Mayo score; n, number of patients achieving each endpoint; N, number of patients in each treatment group; NHI, Nancy Histological Index; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis.
Disclosures:
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Charlie Lees: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Dr Falk – Consultant, Speakers Bureau. Ferring – Speakers Bureau. Gilead – Consultant, Grant/Research Support. GSK – Consultant. Hospira – Consultant, Speakers Bureau. Iterative Scopes – Consultant. Janssen – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Oshi Health – Consultant. Pfizer inc – Consultant, Speakers Bureau. Pharmacosmos – Consultant. Shire – Speakers Bureau. Takeda – Consultant, Speakers Bureau. Topivert – Consultant. Trellus Health – Consultant. Vifor Pharma – Consultant. Warner Chilcott – Speakers Bureau.
Filip Baert: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Abivax – Consultant. Amgen – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant, Speakers Bureau. Celgene – Consultant. Celltrion – Consultant, Speakers Bureau. Eurogenetics – Grant/Research Support. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Speakers Bureau. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Merck Sharp & Dohme – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Sandoz – Consultant. Takeda – Consultant, Speakers Bureau.
Maria Kudela: Pfizer Inc – Employee, Stock Options.
Abhishek Bhattacharjee: Pfizer Healthcare India Private Ltd. – Employee. Pfizer Inc – Stock Options.
Krisztina Lazin: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Arcangelo Abbatemarco: Pfizer Inc – Employee, Stock Options.
Karolina Wosik: Pfizer Canada Inc – Employee. Pfizer Inc – Stock Options.
John Marshall: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Alimentiv – Advisory Committee/Board Member. Amgen – Advisory Committee/Board Member, Speakers Bureau. AstraZeneca – Advisory Committee/Board Member. Bausch Health – Advisory Committee/Board Member, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Ferring – Advisory Committee/Board Member, Speakers Bureau. Fresenius Kabi – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Lilly – Advisory Committee/Board Member. Lupin – Advisory Committee/Board Member. Organon – Advisory Committee/Board Member, Speakers Bureau. Paladin – Advisory Committee/Board Member. Pfizer Inc – Advisory Committee/Board Member, Speakers Bureau. Pharmascience – Advisory Committee/Board Member, Speakers Bureau. Qu Biologics – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member, Speakers Bureau. Sandoz – Advisory Committee/Board Member. SCOPE – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Speakers Bureau. Teva – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member, Speakers Bureau.
David T. Rubin, MD1, Charlie W. Lees, PhD2, Filip Baert, MD3, Maria Kudela, PhD4, Abhishek Bhattacharjee, PhD5, Krisztina Lazin, MD6, Martina Goetsch, MD6, Arcangelo M. Abbatemarco, MD7, Karolina Wosik, MSc, PhD8, John K. Marshall, MSc, MD, BA9. P1071 - Efficacy of Etrasimod as First-Line Advanced Treatment Following Failure of Aminosalicylates Only: Data From the ELEVATE UC 52 Phase 3 Clinical Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 2Institute of Genetics & Molecular Medicine, University of Edinburgh, Edinburgh, UK; Edinburgh IBD Unit, Western General Hospital, NHS Lothian, Edinburgh, UK, Edinburgh, Scotland, United Kingdom; 3Department of Gastroenterology, AZ Delta, Roeselare, Belgium, Roeselare, West-Vlaanderen, Belgium; 4Pfizer Inc, Cambridge, MA, USA, Cambridge, MA; 5Pfizer Healthcare India Private Ltd, Chennai, India, Chennai, Tamil Nadu, India; 6Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 7Pfizer Inc, New York, NY, USA, New York, NY; 8Pfizer Canada, Kirkland, PQ, Canada; 9Division of Gastroenterology, Department of Medicine, Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, ON, Canada, Hamilton, ON, Canada
Introduction: Etrasimod is an oral, once-daily, selective sphingosine 1‑phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). In patients with moderately to severely active UC, treatment guidelines recommend early use of advanced therapies rather than gradual step up after failure of 5-aminosalicylate (5-ASA).1
Methods: This prespecified subgroup analysis assessed the efficacy of etrasimod vs placebo in patients in ELEVATE UC 52 (NCT03945188) with, and without, prior oral 5-ASA failure only (defined as inadequate response, loss of response or intolerance to previous treatment with oral 5-ASA but not to any other previous UC medication). Efficacy endpoints assessed at Weeks 12 and 52 included clinical remission, endoscopic improvement, symptomatic remission and composite histological endpoints.
Results: In total, 45 and 29 patients receiving etrasimod and placebo, respectively, had prior 5-ASA failure only and 244 and 115 patients did not. At Weeks 12 and 52, among those who had failed prior 5-ASA only, significantly more patients receiving etrasimod vs placebo achieved clinical remission (Week 12, % difference [Δ]: 38.3%, p < 0.001; Week 52, Δ: 45.0%, p < 0.001), endoscopic improvement (Week 12, Δ: 54.7%, p < 0.001; Week 52, Δ: 47.8%, p < 0.001), symptomatic remission (Week 12, Δ: 38.6%, p < 0.001; Week 52, Δ: 45.0%, p < 0.001), and endoscopic improvement-histologic remission (Week 12, Δ: 35.6%, p < 0.001; Week 52, Δ: 31.1%, p < 0.001; Table). Significantly more patients receiving etrasimod vs placebo in this subgroup achieved endoscopic normalization-histologic remission (Week 12, Δ: 19.9%, p</em> = 0.002; Week 52, Δ: 24.3%, p < 0.001) and disease clearance (Week 12, Δ: 15.6%, p</em> = 0.008; Week 52, Δ: 27.8%, p < 0.001). Among those who had failed other therapies, significantly more patients receiving etrasimod vs placebo also achieved all endpoints at Weeks 12 and 52 (p < 0.01).
Discussion: Etrasimod demonstrated robust efficacy as first-line advanced therapy in patients who had failed oral 5-ASA only, across all evaluated endpoints including stringent composite histological endpoints, consistent with the overall ELEVATE UC population. Etrasimod shows efficacy when used early in the UC treatment algorithm.
Reference:
1. Singh S et al. Gastroenterology 2024; 167: 1307–1343.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.

Figure: Table. Key efficacy endpoints achieved by patients with prior oral 5-ASA treatment failure only in ELEVATE UC 52.
[a]Clinical remission: SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline), RBS = 0, and ES ≤ 1 (excluding friability).
[b]Difference (95% CI) and 2-sided p value are based on the Cochran–Mantel–Haenszel method adjusting to reported randomization stratification of (1) naïve to biologic or JAK inhibitor therapy at study entry, (2) baseline corticosteroid use, and (3) baseline disease activity (MMS 4–6 or 7–9).
[c]Endoscopic improvement: ES ≤ 1.
[d]Symptomatic remission: SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline) and RBS = 0.
[e]EIHR: ES ≤ 1 with histologic remission (Geboes Index score < 2.0).
[f]Endoscopic normalization-histologic remission: Geboes Index score < 2.0 and ES = 0.
[g]Disease clearance: NHI = 0 and ES = 0 and RBS = 0 and SFS = 0 or 1 (if 1, must have ≥ 1-point decrease from baseline).
5-ASA, 5-aminosalicylate; CI, confidence interval; EIHR, endoscopic improvement-histologic remission; ES, endoscopic subscore; JAK, Janus kinase; MMS, modified Mayo score; n, number of patients achieving each endpoint; N, number of patients in each treatment group; NHI, Nancy Histological Index; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis.
Disclosures:
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Charlie Lees: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Dr Falk – Consultant, Speakers Bureau. Ferring – Speakers Bureau. Gilead – Consultant, Grant/Research Support. GSK – Consultant. Hospira – Consultant, Speakers Bureau. Iterative Scopes – Consultant. Janssen – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Oshi Health – Consultant. Pfizer inc – Consultant, Speakers Bureau. Pharmacosmos – Consultant. Shire – Speakers Bureau. Takeda – Consultant, Speakers Bureau. Topivert – Consultant. Trellus Health – Consultant. Vifor Pharma – Consultant. Warner Chilcott – Speakers Bureau.
Filip Baert: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Abivax – Consultant. Amgen – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant, Speakers Bureau. Celgene – Consultant. Celltrion – Consultant, Speakers Bureau. Eurogenetics – Grant/Research Support. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Speakers Bureau. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Merck Sharp & Dohme – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Sandoz – Consultant. Takeda – Consultant, Speakers Bureau.
Maria Kudela: Pfizer Inc – Employee, Stock Options.
Abhishek Bhattacharjee: Pfizer Healthcare India Private Ltd. – Employee. Pfizer Inc – Stock Options.
Krisztina Lazin: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Arcangelo Abbatemarco: Pfizer Inc – Employee, Stock Options.
Karolina Wosik: Pfizer Canada Inc – Employee. Pfizer Inc – Stock Options.
John Marshall: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Alimentiv – Advisory Committee/Board Member. Amgen – Advisory Committee/Board Member, Speakers Bureau. AstraZeneca – Advisory Committee/Board Member. Bausch Health – Advisory Committee/Board Member, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Ferring – Advisory Committee/Board Member, Speakers Bureau. Fresenius Kabi – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Lilly – Advisory Committee/Board Member. Lupin – Advisory Committee/Board Member. Organon – Advisory Committee/Board Member, Speakers Bureau. Paladin – Advisory Committee/Board Member. Pfizer Inc – Advisory Committee/Board Member, Speakers Bureau. Pharmascience – Advisory Committee/Board Member, Speakers Bureau. Qu Biologics – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member, Speakers Bureau. Sandoz – Advisory Committee/Board Member. SCOPE – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Speakers Bureau. Teva – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member, Speakers Bureau.
David T. Rubin, MD1, Charlie W. Lees, PhD2, Filip Baert, MD3, Maria Kudela, PhD4, Abhishek Bhattacharjee, PhD5, Krisztina Lazin, MD6, Martina Goetsch, MD6, Arcangelo M. Abbatemarco, MD7, Karolina Wosik, MSc, PhD8, John K. Marshall, MSc, MD, BA9. P1071 - Efficacy of Etrasimod as First-Line Advanced Treatment Following Failure of Aminosalicylates Only: Data From the ELEVATE UC 52 Phase 3 Clinical Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
