Sunday Poster Session
Category: IBD
P1058 - Real-World Safety of Sphingosine-1-Phosphate (S1P) Receptor Modulators in Inflammatory Bowel Disease: A FAERS-Based Pharmacovigilance Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- AK
Abhay A. Kapoor, MBBS (he/him/his)
St. Mary Medical Center, Langhorne
Langhorne, PA
Presenting Author(s)
Ratnadeep Biswas, MBBS1, Abhay A. Kapoor, MBBS2, Pratima Jasti, MBBS3, Farheen F. Vhora, MBBS4, Jaahnavee Trivedi, MBBS5
1ESIC Medical College & Hospital, Bihta, Patna, Patna, Bihar, India; 2St. Mary Medical Center, Langhorne, Langhorne, PA; 3All India Institute of Medical Sciences, Bhubaneswar, Bhubaneswar, Orissa, India; 4B.J. Medical College, Ahmedabad, Ahmedabad, Gujarat, India; 5SUNY Downstate Health Sciences University, Brooklyn, NY
Introduction: Sphingosine-1-phosphate (S1P) modulators, including ozanimod and etrasimod, have been approved as novel therapies for inflammatory bowel disease (IBD), particularly ulcerative colitis (UC). Although phase III trials demonstrated efficacy, real-world safety data remain limited. We analyzed the FDA Adverse Event Reporting System (FAERS) to characterize adverse events (AEs) in IBD and identify factors predicting serious AEs.
Methods: FAERS cases through May 2025 for ozanimod or etrasimod with IBD indications were retrieved. Reports were classified as serious if they met FDA criteria (life-threatening, hospitalization, disability, or death). System-organ class (SOC) AEs (e.g., gastrointestinal, central nervous system [CNS], infections, musculoskeletal, cardiovascular) were quantified. Univariate and multivariate logistic regression evaluated associations between serious AEs and age, drug type, IBD subtype (UC or Crohn’s disease [CD]), and presence of each SOC-based AE.
Results: Among 1,982 AE reports (ozanimod 1,743; etrasimod 239), 690 (34.8%) were serious and 16 (0.8%) fatal. Mean age was 47.7 years (SD 16.6). The most frequent AEs by SOC were gastrointestinal (n=567; 28.6%), CNS (n=362; 18.3%), infections (n=143; 7.2%), musculoskeletal (n=138; 7.0%), and cardiovascular (n=137; 6.9%). Hemorrhage (n=178; 9.0%) and fatigue (n=172; 8.7%) were the most common individual AEs. In multivariate regression, gastrointestinal AEs (OR 2.033; 95% CI 1.617–2.555; p< 0.001), infections (OR 2.463; 95% CI 1.619–3.747; p< 0.001), and increased age (per year; OR 1.009; 95% CI 1.003–1.016; p=0.004) independently predicted serious AEs. UC was associated with lower odds of serious AEs than CD (OR 0.330; 95% CI 0.184–0.594; p < 0.001). Ozanimod use (vs. etrasimod) was linked to lower odds of serious AEs (OR 0.606; 95% CI 0.426–0.863; p = 0.005).
Discussion: Over one-third of AEs related to ozanimod or etrasimod in IBD were serious, with a 0.8% fatality rate. Gastrointestinal and infectious AEs were common and independently predicted serious outcomes, underscoring the need for vigilant monitoring of GI symptoms and infection risk when prescribing S1P modulators. Patients with UC had significantly lower odds of serious AEs compared to those with CD, suggesting subtype-specific risk stratification may be warranted. Ozanimod demonstrated a more favorable safety profile compared to etrasimod. These findings can guide risk stratification, counseling, and surveillance strategies in IBD management.
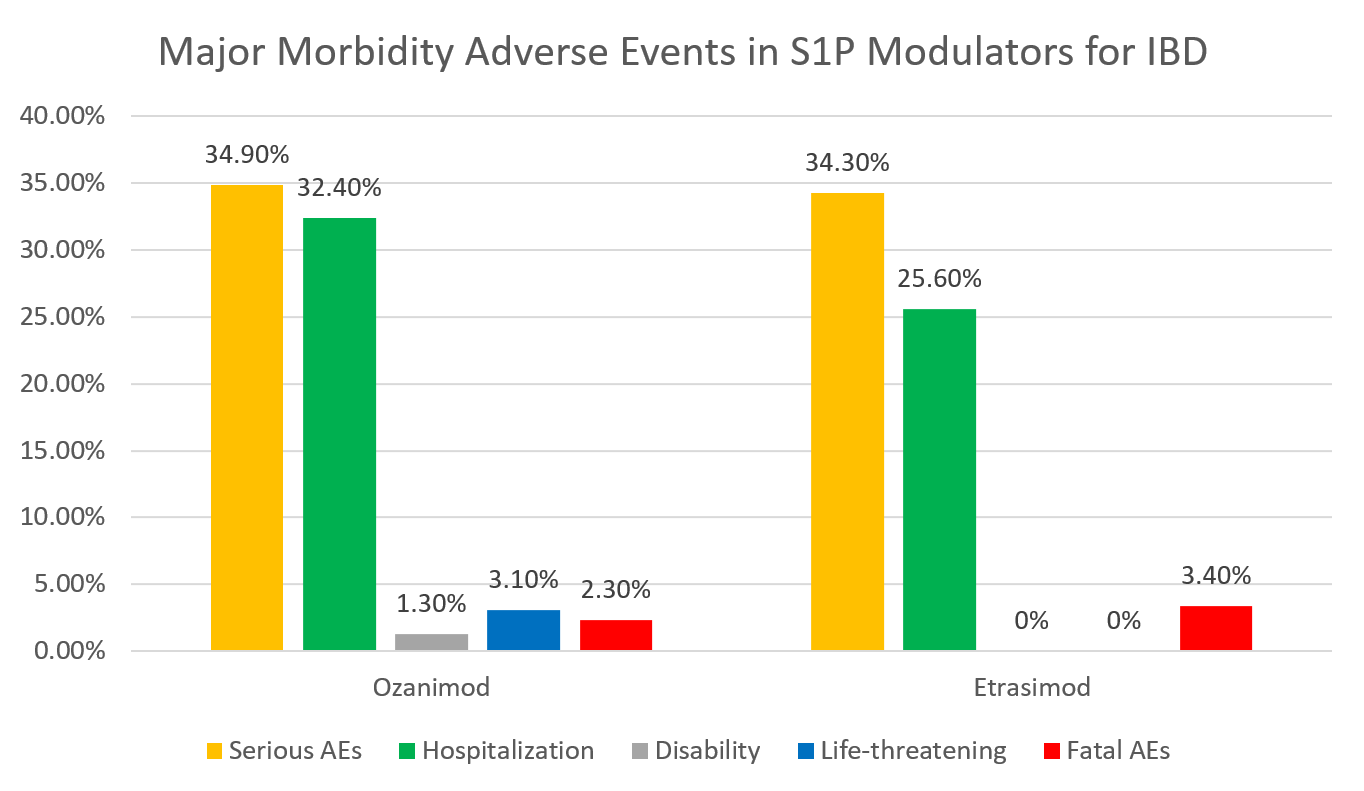
Figure: Distribution of major morbidity adverse events associated with S1P modulators ozanimod and etrasimod in IBD treatment
Disclosures:
Ratnadeep Biswas indicated no relevant financial relationships.
Abhay Kapoor indicated no relevant financial relationships.
Pratima Jasti indicated no relevant financial relationships.
Farheen Vhora indicated no relevant financial relationships.
Jaahnavee Trivedi indicated no relevant financial relationships.
Ratnadeep Biswas, MBBS1, Abhay A. Kapoor, MBBS2, Pratima Jasti, MBBS3, Farheen F. Vhora, MBBS4, Jaahnavee Trivedi, MBBS5. P1058 - Real-World Safety of Sphingosine-1-Phosphate (S1P) Receptor Modulators in Inflammatory Bowel Disease: A FAERS-Based Pharmacovigilance Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1ESIC Medical College & Hospital, Bihta, Patna, Patna, Bihar, India; 2St. Mary Medical Center, Langhorne, Langhorne, PA; 3All India Institute of Medical Sciences, Bhubaneswar, Bhubaneswar, Orissa, India; 4B.J. Medical College, Ahmedabad, Ahmedabad, Gujarat, India; 5SUNY Downstate Health Sciences University, Brooklyn, NY
Introduction: Sphingosine-1-phosphate (S1P) modulators, including ozanimod and etrasimod, have been approved as novel therapies for inflammatory bowel disease (IBD), particularly ulcerative colitis (UC). Although phase III trials demonstrated efficacy, real-world safety data remain limited. We analyzed the FDA Adverse Event Reporting System (FAERS) to characterize adverse events (AEs) in IBD and identify factors predicting serious AEs.
Methods: FAERS cases through May 2025 for ozanimod or etrasimod with IBD indications were retrieved. Reports were classified as serious if they met FDA criteria (life-threatening, hospitalization, disability, or death). System-organ class (SOC) AEs (e.g., gastrointestinal, central nervous system [CNS], infections, musculoskeletal, cardiovascular) were quantified. Univariate and multivariate logistic regression evaluated associations between serious AEs and age, drug type, IBD subtype (UC or Crohn’s disease [CD]), and presence of each SOC-based AE.
Results: Among 1,982 AE reports (ozanimod 1,743; etrasimod 239), 690 (34.8%) were serious and 16 (0.8%) fatal. Mean age was 47.7 years (SD 16.6). The most frequent AEs by SOC were gastrointestinal (n=567; 28.6%), CNS (n=362; 18.3%), infections (n=143; 7.2%), musculoskeletal (n=138; 7.0%), and cardiovascular (n=137; 6.9%). Hemorrhage (n=178; 9.0%) and fatigue (n=172; 8.7%) were the most common individual AEs. In multivariate regression, gastrointestinal AEs (OR 2.033; 95% CI 1.617–2.555; p< 0.001), infections (OR 2.463; 95% CI 1.619–3.747; p< 0.001), and increased age (per year; OR 1.009; 95% CI 1.003–1.016; p=0.004) independently predicted serious AEs. UC was associated with lower odds of serious AEs than CD (OR 0.330; 95% CI 0.184–0.594; p < 0.001). Ozanimod use (vs. etrasimod) was linked to lower odds of serious AEs (OR 0.606; 95% CI 0.426–0.863; p = 0.005).
Discussion: Over one-third of AEs related to ozanimod or etrasimod in IBD were serious, with a 0.8% fatality rate. Gastrointestinal and infectious AEs were common and independently predicted serious outcomes, underscoring the need for vigilant monitoring of GI symptoms and infection risk when prescribing S1P modulators. Patients with UC had significantly lower odds of serious AEs compared to those with CD, suggesting subtype-specific risk stratification may be warranted. Ozanimod demonstrated a more favorable safety profile compared to etrasimod. These findings can guide risk stratification, counseling, and surveillance strategies in IBD management.

Figure: Distribution of major morbidity adverse events associated with S1P modulators ozanimod and etrasimod in IBD treatment
Disclosures:
Ratnadeep Biswas indicated no relevant financial relationships.
Abhay Kapoor indicated no relevant financial relationships.
Pratima Jasti indicated no relevant financial relationships.
Farheen Vhora indicated no relevant financial relationships.
Jaahnavee Trivedi indicated no relevant financial relationships.
Ratnadeep Biswas, MBBS1, Abhay A. Kapoor, MBBS2, Pratima Jasti, MBBS3, Farheen F. Vhora, MBBS4, Jaahnavee Trivedi, MBBS5. P1058 - Real-World Safety of Sphingosine-1-Phosphate (S1P) Receptor Modulators in Inflammatory Bowel Disease: A FAERS-Based Pharmacovigilance Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
