Sunday Poster Session
Category: IBD
P1031 - Glucagon-Like Peptide-1 Receptor Agonists in Inflammatory Bowel Disease: A Comparison of Agent-Specific Outcomes in Type 2 Diabetes and Obese Inflammatory Bowel Disease Populations
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Himaben Gohil, DO, MSc (she/her/hers)
University at Buffalo
Buffalo, NY
Presenting Author(s)
Himaben Gohil, DO, MSc1, Lan Nguyen, DO1, Xavier Zonna, DO1, Randy Cheung, MD2, Erin Ly, MD1
1University at Buffalo, Buffalo, NY; 2University of Buffalo, Buffalo, NY
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) may have anti-inflammatory effects in inflammatory bowel disease (IBD) patients. This has prompted an interest in using GLP-1RAs in IBD patients with obesity or type 2 diabetes mellitus (T2DM). Most studies, however, evaluated GLP-1RAs as a class, limiting insight into agent-specific outcomes. To address this, we conducted a retrospective study comparing 5 U.S Food and Drug Administration approved GLP-1RAs in IBD patients with obesity or T2DM.
Methods: A retrospective study was conducted using TriNetX, a global database of healthcare organizations. We identified adults (≥18 years) with IBD and either obesity or T2DM. Two cohorts were defined: IBD with obesity and IBD with T2DM. Each included a control group without GLP-1RAs exposure and treatment groups receiving dulaglutide, liraglutide, tirzepatide, exenatide, or semaglutide. Patients on multiple GLP-1RAs or chronic steroids were excluded. Treatment groups were propensity score matched to control on demographics, co-morbidities, medications, and inflammatory markers. Body mass index and A1C were also matched in the obesity and T2DM cohorts, respectively. Outcomes included steroid use, hospitalization, IBD-related surgery, and death. Results of outcomes in the obese (Table A) and T2DM cohort (Table B) are shown as risk differences, calculated as percent risk of outcome in control minus treatment group.
Results: Tirzepatide had the greatest reductions in all outcomes and cohorts. Semaglutide also had significant risk reduction in all outcomes but less than tirzepatide. Dulaglutide improved outcomes across cohorts but to a lesser extent than tirzepatide and semaglutide. Notably, the benefits of both semaglutide and dulaglutide were more pronounced in T2DM cohort. Liraglutide offered weak mortality benefit in obesity, increased steroid use in T2DM, and had the smallest reductions in hospitalization and surgery risk across cohorts. Exenatide offered the least benefit, with the poorest mortality profile and increased steroid use in both cohorts.
Discussion: Tirzepatide outperformed all other agents. Semaglutide and dulaglutide were also beneficial, especially in T2DM cohort. In contrast, exenatide and liraglutide should be used with caution due to their limited and unfavorable effects. These findings challenge the assumption of a class effect of GLP-1RAs. Future research should assess GLP1-RAs across IBD phenotypes and metabolic subtypes to guide further agent-specific treatments.
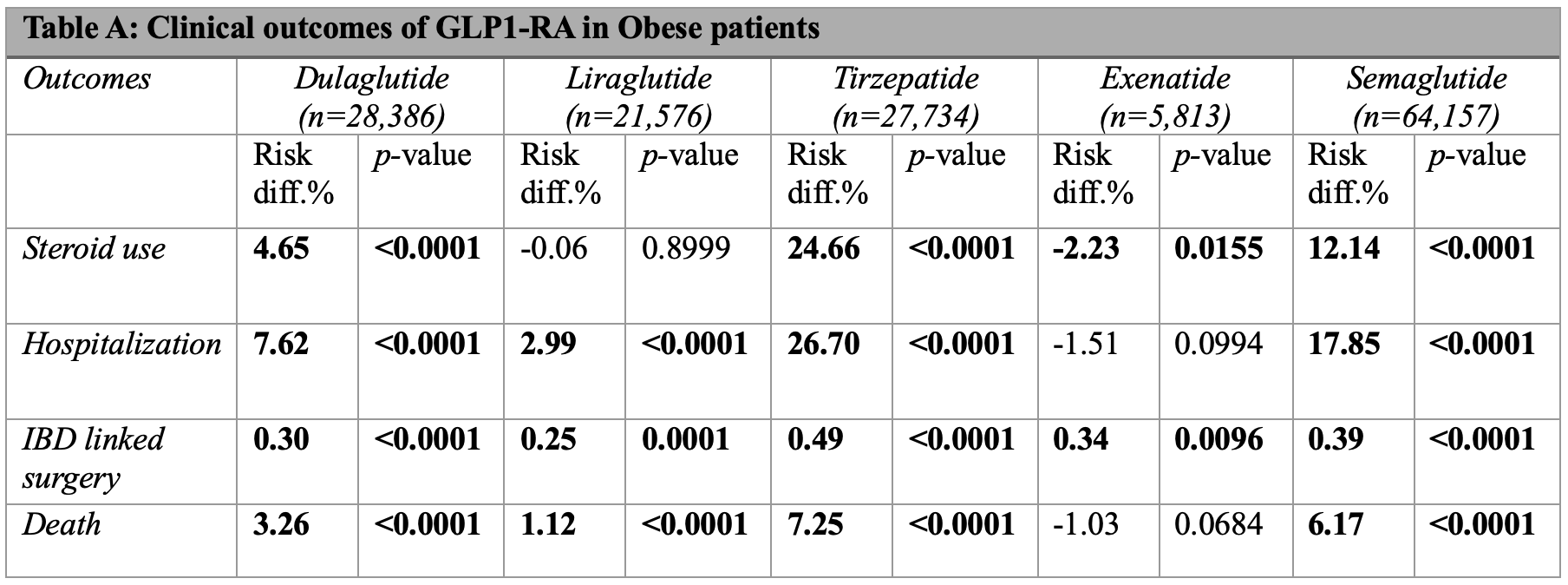
Figure: Table A: Clinical outcomes of GLP-1RA in Obese IBD patients. Results shown as risk difference, calculated as percent risk of a outcome in control group minus treatment group. Bolded numbers indicate statistical significance (p < 0.05).
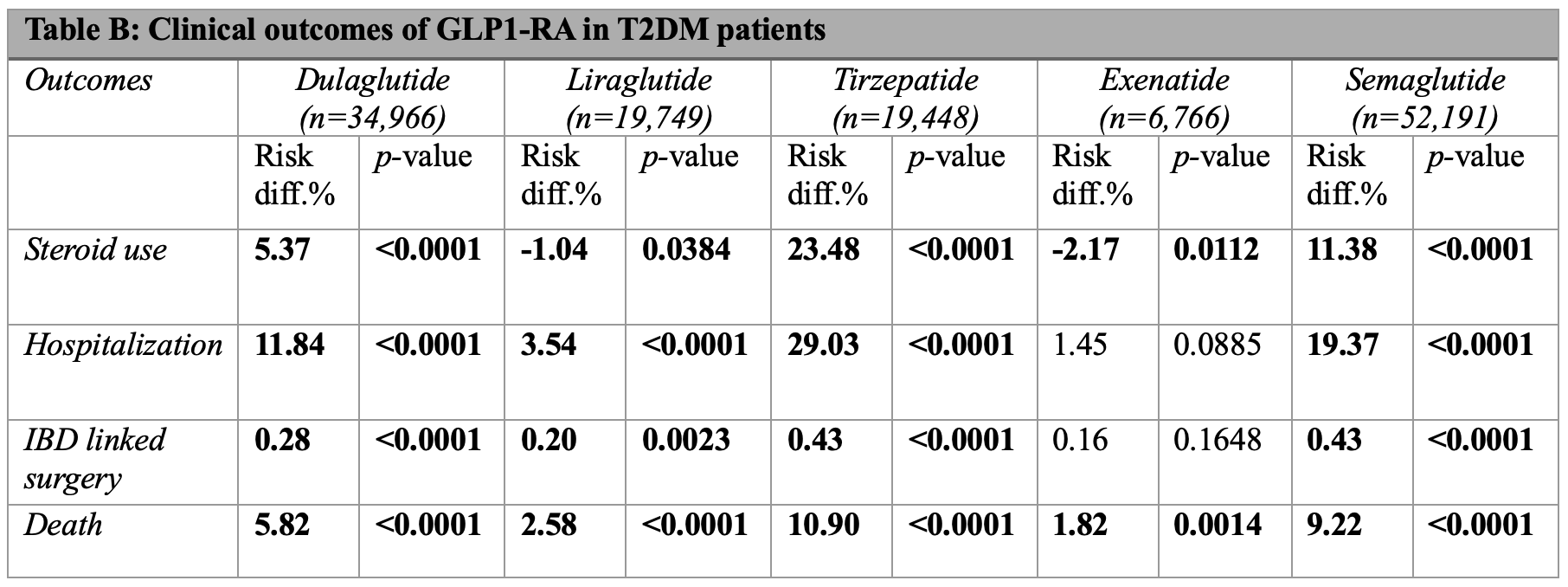
Figure: Table B: Clinical outcomes of GLP-1RA in T2DM IBD patients. Results shown as risk difference, calculated as percent risk of a outcome in control group minus treatment group. Bolded numbers indicate statistical significance (p < 0.05).
Disclosures:
Himaben Gohil indicated no relevant financial relationships.
Lan Nguyen indicated no relevant financial relationships.
Xavier Zonna indicated no relevant financial relationships.
Randy Cheung indicated no relevant financial relationships.
Erin Ly indicated no relevant financial relationships.
Himaben Gohil, DO, MSc1, Lan Nguyen, DO1, Xavier Zonna, DO1, Randy Cheung, MD2, Erin Ly, MD1. P1031 - Glucagon-Like Peptide-1 Receptor Agonists in Inflammatory Bowel Disease: A Comparison of Agent-Specific Outcomes in Type 2 Diabetes and Obese Inflammatory Bowel Disease Populations, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University at Buffalo, Buffalo, NY; 2University of Buffalo, Buffalo, NY
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) may have anti-inflammatory effects in inflammatory bowel disease (IBD) patients. This has prompted an interest in using GLP-1RAs in IBD patients with obesity or type 2 diabetes mellitus (T2DM). Most studies, however, evaluated GLP-1RAs as a class, limiting insight into agent-specific outcomes. To address this, we conducted a retrospective study comparing 5 U.S Food and Drug Administration approved GLP-1RAs in IBD patients with obesity or T2DM.
Methods: A retrospective study was conducted using TriNetX, a global database of healthcare organizations. We identified adults (≥18 years) with IBD and either obesity or T2DM. Two cohorts were defined: IBD with obesity and IBD with T2DM. Each included a control group without GLP-1RAs exposure and treatment groups receiving dulaglutide, liraglutide, tirzepatide, exenatide, or semaglutide. Patients on multiple GLP-1RAs or chronic steroids were excluded. Treatment groups were propensity score matched to control on demographics, co-morbidities, medications, and inflammatory markers. Body mass index and A1C were also matched in the obesity and T2DM cohorts, respectively. Outcomes included steroid use, hospitalization, IBD-related surgery, and death. Results of outcomes in the obese (Table A) and T2DM cohort (Table B) are shown as risk differences, calculated as percent risk of outcome in control minus treatment group.
Results: Tirzepatide had the greatest reductions in all outcomes and cohorts. Semaglutide also had significant risk reduction in all outcomes but less than tirzepatide. Dulaglutide improved outcomes across cohorts but to a lesser extent than tirzepatide and semaglutide. Notably, the benefits of both semaglutide and dulaglutide were more pronounced in T2DM cohort. Liraglutide offered weak mortality benefit in obesity, increased steroid use in T2DM, and had the smallest reductions in hospitalization and surgery risk across cohorts. Exenatide offered the least benefit, with the poorest mortality profile and increased steroid use in both cohorts.
Discussion: Tirzepatide outperformed all other agents. Semaglutide and dulaglutide were also beneficial, especially in T2DM cohort. In contrast, exenatide and liraglutide should be used with caution due to their limited and unfavorable effects. These findings challenge the assumption of a class effect of GLP-1RAs. Future research should assess GLP1-RAs across IBD phenotypes and metabolic subtypes to guide further agent-specific treatments.

Figure: Table A: Clinical outcomes of GLP-1RA in Obese IBD patients. Results shown as risk difference, calculated as percent risk of a outcome in control group minus treatment group. Bolded numbers indicate statistical significance (p < 0.05).

Figure: Table B: Clinical outcomes of GLP-1RA in T2DM IBD patients. Results shown as risk difference, calculated as percent risk of a outcome in control group minus treatment group. Bolded numbers indicate statistical significance (p < 0.05).
Disclosures:
Himaben Gohil indicated no relevant financial relationships.
Lan Nguyen indicated no relevant financial relationships.
Xavier Zonna indicated no relevant financial relationships.
Randy Cheung indicated no relevant financial relationships.
Erin Ly indicated no relevant financial relationships.
Himaben Gohil, DO, MSc1, Lan Nguyen, DO1, Xavier Zonna, DO1, Randy Cheung, MD2, Erin Ly, MD1. P1031 - Glucagon-Like Peptide-1 Receptor Agonists in Inflammatory Bowel Disease: A Comparison of Agent-Specific Outcomes in Type 2 Diabetes and Obese Inflammatory Bowel Disease Populations, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
