Sunday Poster Session
Category: IBD
P1026 - Gut Microbiome Dysbiosis as a Diagnostic Signature in Early Inflammatory Bowel Disease
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Kusalik Boppana, MBBS (he/him/his)
Kasturba Medical College of Manipal
Cranbury, NJ
Presenting Author(s)
Kusalik Boppana, MBBS1, Ashish Chilakapati, MBBS2, Vinay Chandra Pokala, MBBS3, Nayanika Tummala, MD4, Tavishi Katoch, MD5, Binay Panjiyar, MD6, Manisha Chavan, MBBS7
1Kasturba Medical College of Manipal, Cranbury, NJ; 2MBBS, Tampa, FL; 3shri BM patil medical college, Hyderabad, Telangana, India; 4St. Mary's General Hospital, New York Medical College, Poughkeepsie, NY; 5Saint Peter's University Hospital, Greater Noida West, Uttar Pradesh, India; 6NorthShore University Hospital, Manhasset, NY; 7Kakatiya medical college, Oshawa, ON, Canada
Introduction: Inflammatory Bowel Disease (IBD), encompassing Ulcerative Colitis (UC) and Crohn’s Disease (CD), is a persistent inflammatory condition of the digestive tract that is increasing in prevalence worldwide. Early diagnosis remains a challenge, limiting timely intervention. Growing research indicates that gut microbial dysbiosis may precede clinical onset, offering promise as a non-invasive biomarker. This review aims to assess microbiome alterations during early or preclinical IBD.
Methods: We conducted a systematic review on the basis of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. As part of this process, we thoroughly searched three major databases-Embase, PubMed, and the Cochrane library for studies that were published between 2018 and 2024. Included studies examined gut microbiota in early or newly diagnosed IBD patients compared to healthy controls. We individually analysed the studies, collected relevant information, and evaluated their quality utilizing the Newcastle-Ottawa Scale and the Cochrane Risk of Bias tool.
Results: Twenty studies involving 4,689 participants (2,713 IBD patients, 1,976 controls) met inclusion criteria. Seventeen studies reported significantly reduced alpha diversity in early IBD, with one meta-analysis noting a combined mean difference of −0.45 (95% CI: −0.65 to −0.29; p< 0.001). Beta diversity analyses revealed distinct microbial community structures in IBD groups (p< 0.01 in 12 studies). Taxonomic analyses showed consistent depletion of beneficial commensals, including Faecalibacterium prausnitzii, Roseburia, and Bacteroidetes, in ≥14 studies. Fourteen studies reported enrichment of potentially pathogenic taxa such as Escherichia coli and Ruminococcus gnavus. Furthermore, twelve studies observed a decreased presence of Blautia and Coprococcus, which yield short-chain fatty acids (SCFAs), in early Crohn’s disease, with statistical significance (p < 0.05).
Discussion: This review highlights consistent patterns of microbial dysbiosis in early IBD. The reduction in alpha diversity, loss of anti-inflammatory taxa, and increase in pathobionts precede symptom onset, suggesting their utility as early diagnostic markers. Identifying these microbial signatures offers potential for non-invasive, preclinical screening and risk stratification strategies. Additional longitudinal research is necessary to confirm these biomarkers and incorporate microbiome profiling into individualized preventive healthcare.

Figure: Microbial signatures associated with early Inflammatory Bowel Disease
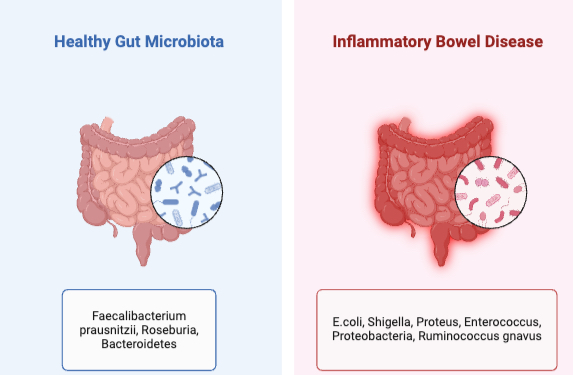
Figure: Microbial composition differences between healthy gut and Inflammatory Bowel Disease patients
Disclosures:
Kusalik Boppana indicated no relevant financial relationships.
Ashish Chilakapati indicated no relevant financial relationships.
Vinay Chandra Pokala indicated no relevant financial relationships.
Nayanika Tummala indicated no relevant financial relationships.
Tavishi Katoch indicated no relevant financial relationships.
Binay Panjiyar indicated no relevant financial relationships.
Manisha Chavan indicated no relevant financial relationships.
Kusalik Boppana, MBBS1, Ashish Chilakapati, MBBS2, Vinay Chandra Pokala, MBBS3, Nayanika Tummala, MD4, Tavishi Katoch, MD5, Binay Panjiyar, MD6, Manisha Chavan, MBBS7. P1026 - Gut Microbiome Dysbiosis as a Diagnostic Signature in Early Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Kasturba Medical College of Manipal, Cranbury, NJ; 2MBBS, Tampa, FL; 3shri BM patil medical college, Hyderabad, Telangana, India; 4St. Mary's General Hospital, New York Medical College, Poughkeepsie, NY; 5Saint Peter's University Hospital, Greater Noida West, Uttar Pradesh, India; 6NorthShore University Hospital, Manhasset, NY; 7Kakatiya medical college, Oshawa, ON, Canada
Introduction: Inflammatory Bowel Disease (IBD), encompassing Ulcerative Colitis (UC) and Crohn’s Disease (CD), is a persistent inflammatory condition of the digestive tract that is increasing in prevalence worldwide. Early diagnosis remains a challenge, limiting timely intervention. Growing research indicates that gut microbial dysbiosis may precede clinical onset, offering promise as a non-invasive biomarker. This review aims to assess microbiome alterations during early or preclinical IBD.
Methods: We conducted a systematic review on the basis of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. As part of this process, we thoroughly searched three major databases-Embase, PubMed, and the Cochrane library for studies that were published between 2018 and 2024. Included studies examined gut microbiota in early or newly diagnosed IBD patients compared to healthy controls. We individually analysed the studies, collected relevant information, and evaluated their quality utilizing the Newcastle-Ottawa Scale and the Cochrane Risk of Bias tool.
Results: Twenty studies involving 4,689 participants (2,713 IBD patients, 1,976 controls) met inclusion criteria. Seventeen studies reported significantly reduced alpha diversity in early IBD, with one meta-analysis noting a combined mean difference of −0.45 (95% CI: −0.65 to −0.29; p< 0.001). Beta diversity analyses revealed distinct microbial community structures in IBD groups (p< 0.01 in 12 studies). Taxonomic analyses showed consistent depletion of beneficial commensals, including Faecalibacterium prausnitzii, Roseburia, and Bacteroidetes, in ≥14 studies. Fourteen studies reported enrichment of potentially pathogenic taxa such as Escherichia coli and Ruminococcus gnavus. Furthermore, twelve studies observed a decreased presence of Blautia and Coprococcus, which yield short-chain fatty acids (SCFAs), in early Crohn’s disease, with statistical significance (p < 0.05).
Discussion: This review highlights consistent patterns of microbial dysbiosis in early IBD. The reduction in alpha diversity, loss of anti-inflammatory taxa, and increase in pathobionts precede symptom onset, suggesting their utility as early diagnostic markers. Identifying these microbial signatures offers potential for non-invasive, preclinical screening and risk stratification strategies. Additional longitudinal research is necessary to confirm these biomarkers and incorporate microbiome profiling into individualized preventive healthcare.

Figure: Microbial signatures associated with early Inflammatory Bowel Disease

Figure: Microbial composition differences between healthy gut and Inflammatory Bowel Disease patients
Disclosures:
Kusalik Boppana indicated no relevant financial relationships.
Ashish Chilakapati indicated no relevant financial relationships.
Vinay Chandra Pokala indicated no relevant financial relationships.
Nayanika Tummala indicated no relevant financial relationships.
Tavishi Katoch indicated no relevant financial relationships.
Binay Panjiyar indicated no relevant financial relationships.
Manisha Chavan indicated no relevant financial relationships.
Kusalik Boppana, MBBS1, Ashish Chilakapati, MBBS2, Vinay Chandra Pokala, MBBS3, Nayanika Tummala, MD4, Tavishi Katoch, MD5, Binay Panjiyar, MD6, Manisha Chavan, MBBS7. P1026 - Gut Microbiome Dysbiosis as a Diagnostic Signature in Early Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
