Sunday Poster Session
Category: IBD
P1023 - Real-World Drug Persistence and Clinical Effectiveness of Ustekinumab After Dual Anti-TNF Failure in Crohn’s Disease
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Carl Samaha, MD
University of Pittsburgh
Pittsburgh, PA
Presenting Author(s)
Carl Samaha, MD1, Logan Slade, PA1, Jeffrey M. Dueker, MD, MPH1, Elyse Johnston, MD1, David Binion, MD2, Marc Schwartz, MD1, Richard Duerr, MD1
1University of Pittsburgh, Pittsburgh, PA; 2University of Pittsburgh Medical Center, Pittsburgh, PA
Introduction: Patients with Crohn’s Disease (CD) frequently cycle through biologic therapies despite initial response. Clinical trials and real-world studies have demonstrated the efficacy of Ustekinumab (UST), an IL-12/23 inhibitor, as second-line therapy after trial of an anti-TNF. However, real-world data remain limited in its long-term use after dual anti-TNF failure. This study evaluated the persistence and clinical effectiveness of UST in an anti-TNF refractory CD cohort.
Methods: We retrospectively analyzed a cohort of anti-TNF refractory patients with CD enrolled in the SPARC-IBD study. Anti-TNF failure was defined as primary non-response, loss of response, or intolerance due to delayed side effects. We identified 30 patients who initiated UST between 2018 and 2023 following documented failure of ≥2 anti-TNF agents and retrieved their baseline demographics, disease phenotype, and prior medical and surgical history. The primary outcome was UST persistence evaluated by Kaplan-Meier survival analysis. The secondary outcomes were clinical and endoscopic remission measured by sCDAI and SES-CD.
Results: The cohort was majority female (70%) with a median age of 40.5. Most patients had stricturing/fistulizing disease (73%); perianal disease (50%), upper GI involvement (40%), and a history of ileocecal resection (77%) were prevalent. Nearly all had received infliximab (IFX) and adalimumab (ADA) prior to initiating UST. Discontinuation of anti-TNF was due to primary nonresponse (35%), loss of response (27%), and intolerance – infusion/injection reactions (16%), lupus-like syndromes (13%) or psoriasiform rashes (10%). Ten patients (33.3%) discontinued UST during follow-up. The median drug persistence was 2304 days (~6.3 years). At 12 months, over 90% of patients remained on treatment compared to 70% at 3 years and only 43% at 6 years (Figure 1). Among patients still receiving UST at last follow-up (n=20), 70% were in clinical remission and 79% in endoscopic remission, compared to 20% and 21% with mild activity, and 10% with moderate clinical activity.
Discussion: In this real-world cohort of patients with CD refractory to anti-TNF therapy, UST demonstrated high persistence and efficacy through 1 and 3 years. However, more than half of patients ultimately discontinued therapy by six years, reflecting on the challenge of maintaining long-term biologics use in refractory disease. These finding highlight the clinical value of UST after anti-TNF therapy and the need for optimized maintenance strategies.
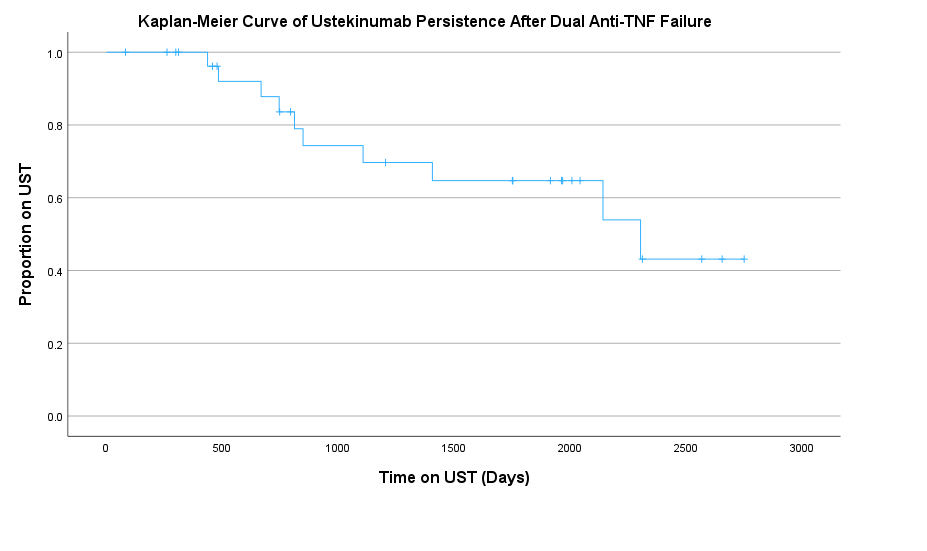
Figure: Figure 1: Kaplan-Meir Curve of Ustekinumab Survival after Dual Anti-TNF Failure
Disclosures:
Carl Samaha indicated no relevant financial relationships.
Logan Slade indicated no relevant financial relationships.
Jeffrey Dueker indicated no relevant financial relationships.
Elyse Johnston indicated no relevant financial relationships.
David Binion: Blueprint Medicines – Grant/Research Support. Mirador Therapeutics – Grant/Research Support. Takeda – Grant/Research Support.
Marc Schwartz indicated no relevant financial relationships.
Richard Duerr indicated no relevant financial relationships.
Carl Samaha, MD1, Logan Slade, PA1, Jeffrey M. Dueker, MD, MPH1, Elyse Johnston, MD1, David Binion, MD2, Marc Schwartz, MD1, Richard Duerr, MD1. P1023 - Real-World Drug Persistence and Clinical Effectiveness of Ustekinumab After Dual Anti-TNF Failure in Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Pittsburgh, Pittsburgh, PA; 2University of Pittsburgh Medical Center, Pittsburgh, PA
Introduction: Patients with Crohn’s Disease (CD) frequently cycle through biologic therapies despite initial response. Clinical trials and real-world studies have demonstrated the efficacy of Ustekinumab (UST), an IL-12/23 inhibitor, as second-line therapy after trial of an anti-TNF. However, real-world data remain limited in its long-term use after dual anti-TNF failure. This study evaluated the persistence and clinical effectiveness of UST in an anti-TNF refractory CD cohort.
Methods: We retrospectively analyzed a cohort of anti-TNF refractory patients with CD enrolled in the SPARC-IBD study. Anti-TNF failure was defined as primary non-response, loss of response, or intolerance due to delayed side effects. We identified 30 patients who initiated UST between 2018 and 2023 following documented failure of ≥2 anti-TNF agents and retrieved their baseline demographics, disease phenotype, and prior medical and surgical history. The primary outcome was UST persistence evaluated by Kaplan-Meier survival analysis. The secondary outcomes were clinical and endoscopic remission measured by sCDAI and SES-CD.
Results: The cohort was majority female (70%) with a median age of 40.5. Most patients had stricturing/fistulizing disease (73%); perianal disease (50%), upper GI involvement (40%), and a history of ileocecal resection (77%) were prevalent. Nearly all had received infliximab (IFX) and adalimumab (ADA) prior to initiating UST. Discontinuation of anti-TNF was due to primary nonresponse (35%), loss of response (27%), and intolerance – infusion/injection reactions (16%), lupus-like syndromes (13%) or psoriasiform rashes (10%). Ten patients (33.3%) discontinued UST during follow-up. The median drug persistence was 2304 days (~6.3 years). At 12 months, over 90% of patients remained on treatment compared to 70% at 3 years and only 43% at 6 years (Figure 1). Among patients still receiving UST at last follow-up (n=20), 70% were in clinical remission and 79% in endoscopic remission, compared to 20% and 21% with mild activity, and 10% with moderate clinical activity.
Discussion: In this real-world cohort of patients with CD refractory to anti-TNF therapy, UST demonstrated high persistence and efficacy through 1 and 3 years. However, more than half of patients ultimately discontinued therapy by six years, reflecting on the challenge of maintaining long-term biologics use in refractory disease. These finding highlight the clinical value of UST after anti-TNF therapy and the need for optimized maintenance strategies.

Figure: Figure 1: Kaplan-Meir Curve of Ustekinumab Survival after Dual Anti-TNF Failure
Disclosures:
Carl Samaha indicated no relevant financial relationships.
Logan Slade indicated no relevant financial relationships.
Jeffrey Dueker indicated no relevant financial relationships.
Elyse Johnston indicated no relevant financial relationships.
David Binion: Blueprint Medicines – Grant/Research Support. Mirador Therapeutics – Grant/Research Support. Takeda – Grant/Research Support.
Marc Schwartz indicated no relevant financial relationships.
Richard Duerr indicated no relevant financial relationships.
Carl Samaha, MD1, Logan Slade, PA1, Jeffrey M. Dueker, MD, MPH1, Elyse Johnston, MD1, David Binion, MD2, Marc Schwartz, MD1, Richard Duerr, MD1. P1023 - Real-World Drug Persistence and Clinical Effectiveness of Ustekinumab After Dual Anti-TNF Failure in Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
