Sunday Poster Session
Category: IBD
P1019 - Real-World Comparison of Upadacitinib and Ustekinumab for Ulcerative Colitis: A Propensity-Matched Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Khaled Elfert, MBChB, MRCP
West Virginia University School of Medicine
Morgantown, WV
Presenting Author(s)
Khaled Elfert, MBChB, MRCP1, Mohamed Eldesouki, MD2, Hamza Almusabeh, MBBS1, Esraa Elromisy, MBChB3, Jennifer Hadam-Veverka, MD1
1West Virginia University School of Medicine, Morgantown, WV; 2Saint Michael's Medical Center, New York Medical College, Newark, NJ; 3Tanta University, Morgantown, WV
Introduction: Upadacitinib (UPA), a selective JAK1 inhibitor, and Ustekinumab (USTE), an interleukin-12/23 inhibitor, are approved for the treatment of moderate-to-severe ulcerative colitis (UC). Real-world head-to-head comparative data between these therapies remain limited.
Methods: We conducted a retrospective cohort study using the TriNetX US Collaborative Network, a federated electronic health record database. Adult patients with UC who were on UPA or USTE between April 2022 and April 2024 were identified. Patients were excluded for co-diagnoses of Crohn’s disease or if they previously underwent total colectomy. Propensity score matching (1:1) was performed on demographics, prior medication use (prednisone, azathioprine, mesalamine, infliximab, adalimumab), and laboratory values (albumin, CRP, fecal calprotectin). The primary outcome was systemic corticosteroids use. Secondary outcomes included emergency department (ED) visit and composite outcome of major cardiovascular events or thrombosis (pulmonary embolism and deep vein thrombosis).
Results: A total of 1,169 patients in the UPA cohort and 2,680 in the USTE cohort met inclusion criteria. After matching, 1,146 patients remained in each cohort, with balanced baseline characteristics. At 1-year follow-up, the primary outcome (corticosteroid use) occurred in 39.4% of patients in the UPA group and 43.7% in the USTE group (RR=0.90, 95% CI: 0.82–0.99, p=0.038). ED visits occurred in 14.5% vs. 12.9%, respectively (RR=1.12, 95% CI: 0.91–1.34, p=0.27). The composite outcome of major cardiovascular events or thrombosis occurred in 1.29% vs. 0.95% (RR=1.37, 95% CI: 0.61,3.063, P=0.4464).
Discussion: In this real-world analysis, treatment with Upadacitinib was associated with lower rates of corticosteroid use compared to Ustekinumab in patients with ulcerative colitis over 1 year. There was no statistically significant difference in the emergency department admissions. These findings indicates potential higher effectiveness of Upadacitinib in UC compared to Ustekinumab and may inform treatment selection.
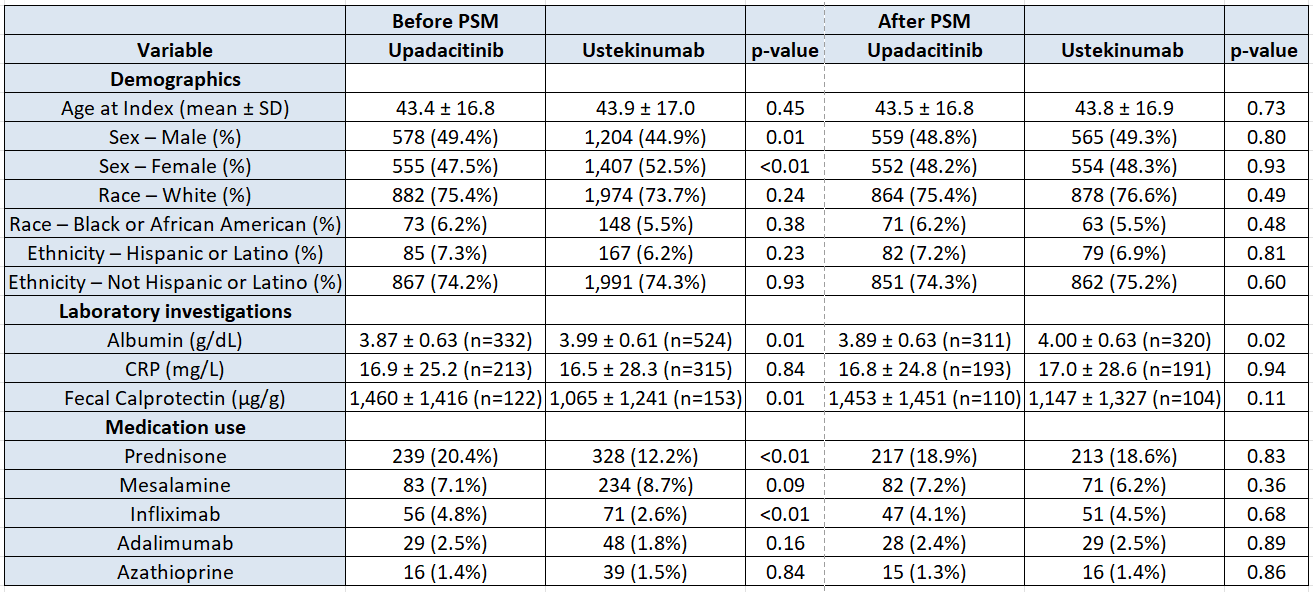
Figure: Baseline characteristics of Upadacitinib and Ustekinumab groups before and after propensity score matching (PSM).
Disclosures:
Khaled Elfert indicated no relevant financial relationships.
Mohamed Eldesouki indicated no relevant financial relationships.
Hamza Almusabeh indicated no relevant financial relationships.
Esraa Elromisy indicated no relevant financial relationships.
Jennifer Hadam-Veverka: Abbvie – Speakers Bureau. Johnson & Johnson – Speakers Bureau. Takeda – Speakers Bureau.
Khaled Elfert, MBChB, MRCP1, Mohamed Eldesouki, MD2, Hamza Almusabeh, MBBS1, Esraa Elromisy, MBChB3, Jennifer Hadam-Veverka, MD1. P1019 - Real-World Comparison of Upadacitinib and Ustekinumab for Ulcerative Colitis: A Propensity-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1West Virginia University School of Medicine, Morgantown, WV; 2Saint Michael's Medical Center, New York Medical College, Newark, NJ; 3Tanta University, Morgantown, WV
Introduction: Upadacitinib (UPA), a selective JAK1 inhibitor, and Ustekinumab (USTE), an interleukin-12/23 inhibitor, are approved for the treatment of moderate-to-severe ulcerative colitis (UC). Real-world head-to-head comparative data between these therapies remain limited.
Methods: We conducted a retrospective cohort study using the TriNetX US Collaborative Network, a federated electronic health record database. Adult patients with UC who were on UPA or USTE between April 2022 and April 2024 were identified. Patients were excluded for co-diagnoses of Crohn’s disease or if they previously underwent total colectomy. Propensity score matching (1:1) was performed on demographics, prior medication use (prednisone, azathioprine, mesalamine, infliximab, adalimumab), and laboratory values (albumin, CRP, fecal calprotectin). The primary outcome was systemic corticosteroids use. Secondary outcomes included emergency department (ED) visit and composite outcome of major cardiovascular events or thrombosis (pulmonary embolism and deep vein thrombosis).
Results: A total of 1,169 patients in the UPA cohort and 2,680 in the USTE cohort met inclusion criteria. After matching, 1,146 patients remained in each cohort, with balanced baseline characteristics. At 1-year follow-up, the primary outcome (corticosteroid use) occurred in 39.4% of patients in the UPA group and 43.7% in the USTE group (RR=0.90, 95% CI: 0.82–0.99, p=0.038). ED visits occurred in 14.5% vs. 12.9%, respectively (RR=1.12, 95% CI: 0.91–1.34, p=0.27). The composite outcome of major cardiovascular events or thrombosis occurred in 1.29% vs. 0.95% (RR=1.37, 95% CI: 0.61,3.063, P=0.4464).
Discussion: In this real-world analysis, treatment with Upadacitinib was associated with lower rates of corticosteroid use compared to Ustekinumab in patients with ulcerative colitis over 1 year. There was no statistically significant difference in the emergency department admissions. These findings indicates potential higher effectiveness of Upadacitinib in UC compared to Ustekinumab and may inform treatment selection.

Figure: Baseline characteristics of Upadacitinib and Ustekinumab groups before and after propensity score matching (PSM).
Disclosures:
Khaled Elfert indicated no relevant financial relationships.
Mohamed Eldesouki indicated no relevant financial relationships.
Hamza Almusabeh indicated no relevant financial relationships.
Esraa Elromisy indicated no relevant financial relationships.
Jennifer Hadam-Veverka: Abbvie – Speakers Bureau. Johnson & Johnson – Speakers Bureau. Takeda – Speakers Bureau.
Khaled Elfert, MBChB, MRCP1, Mohamed Eldesouki, MD2, Hamza Almusabeh, MBBS1, Esraa Elromisy, MBChB3, Jennifer Hadam-Veverka, MD1. P1019 - Real-World Comparison of Upadacitinib and Ustekinumab for Ulcerative Colitis: A Propensity-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
