Sunday Poster Session
Category: General Endoscopy
P0853 - Glucagon-Like Peptide-1 Receptor Agonists and Upper Endoscopy: A Data-Driven Approach to Pre-Procedural Optimization
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- DN
Danial Nadeem, MD
Geisinger Wyoming Valley Medical Center
Wilkes-Barre, PA
Presenting Author(s)
Danial Nadeem, MD1, Tejas Nikumbh, MD2, Daniel Satterfield, CRNP3, James Dove, MS3, Craig Wood, MS3, Peter Benotti, MD3, Harshit S. Khara, MD4, Amitpal Johal, MD3, Christopher Still, DO3
1Geisinger Wyoming Valley Medical Center, Wilkes-Barre, PA; 2The Wright Center for Graduate Medical Education, Dunmore, PA; 3Geisinger Health System, Danville, PA; 4Geisinger Health System, Danville, NJ
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are commonly used in the treatment of type 2 diabetes mellitus and obesity. However, their effect on delaying gastric emptying can complicate upper endoscopy (EGD), where gastric clearance is critical for procedural safety and visualization. Despite these concerns, formal guidance on peri-procedural GLP-1 RA management has been inconsistent. In response, our institution implemented a standardized protocol requiring a 24-hour clear liquid diet and temporary discontinuation of GLP-1 RAs prior to EGD. This study assessed the protocol’s impact on gastric food retention, procedure cancellations, and repeat EGDs within 30 days.
Methods: We conducted a retrospective cohort study across the Geisinger health system comparing outcomes before (January 2022–August 2023) and after (September 2023–December 2024) implementation of a standardized protocol requiring a 24-hour clear liquid diet and temporary discontinuation of GLP-1 RA medications prior to EGD. Daily-dosed medications were held the morning of the procedure, whereas weekly formulations were held for three days prior.
Adults (≥18 years) with GLP-1 RA use within 90 days of outpatient EGD were included; those with gastroparesis were excluded. The primary outcome was gastric food retention per EGD reports. Secondary outcomes included aborted procedures and repeat EGDs within 30 days. Logistic regression was adjusted for age, sex, BMI, and race/ethnicity. Analyses were stratified by diabetes status.
Results: Among 641 pre-intervention and 554 post-intervention EGDs, the mean patient age was 52 years; 69% were female, and 59% had diabetes. Food retention significantly decreased post-intervention (11.4% vs. 5.1%; 95% CI: 0.27–0.65; p = 0.0001) regardless of diabetes status. Among those with food retention, a higher proportion had only small-volume retention post-intervention (30% vs. 4%; p = 0.0043). Repeat EGDs within 30 days declined significantly (2.5% vs. 0.4%; p = 0.0030) and aborted procedures trended lower (1.4% vs. 0.4%; p = 0.072).
Discussion: Implementation of a GLP-1 RA pre-endoscopy guideline significantly reduced food retention, lowering aspiration risk, procedure cancellations, and unnecessary repeat EGDs. These findings highlight a scalable, cost-effective intervention that improves endoscopic efficiency and may reduce healthcare costs by minimizing anesthesia use, provider time, and operating room resources.
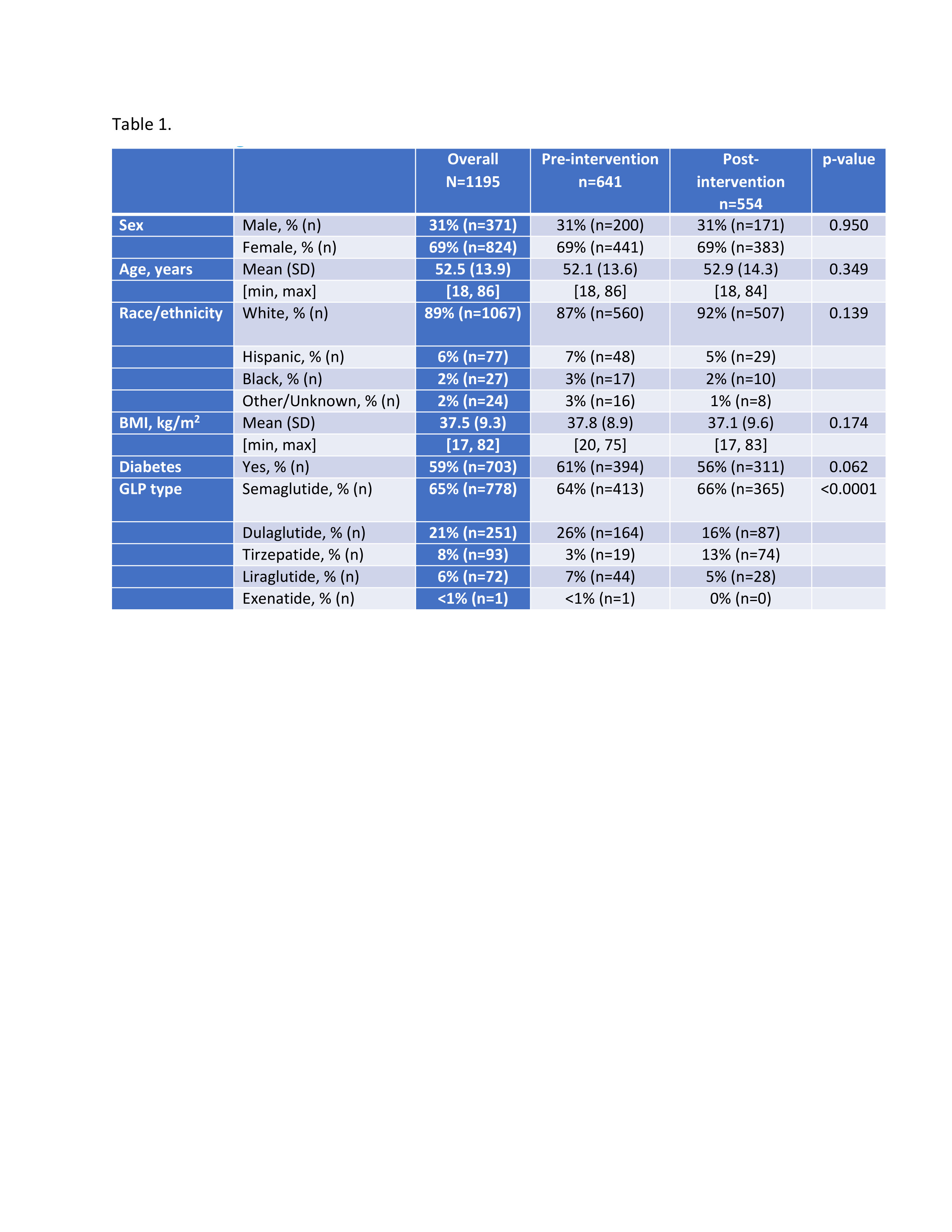
Figure: Table 1. Baseline demographic and clinical characteristics of patients undergoing EGD before and after implementation of GLP-1 RA procedural guidance.
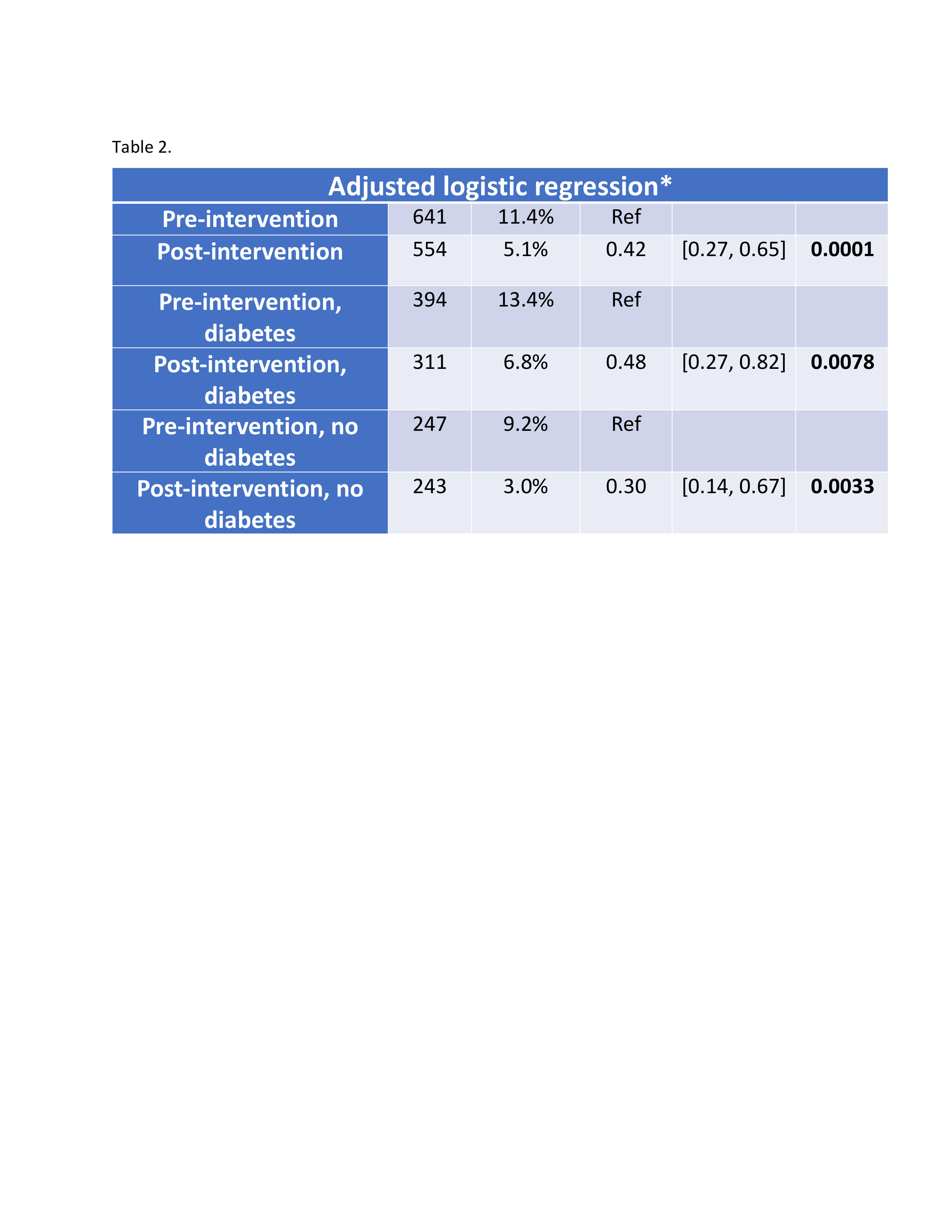
Figure: Table 2. Adjusted logistic regression analysis of gastric content retention rates before and after implementation of GLP-1 RA procedural guidance, stratified by diabetes status.
Disclosures:
Danial Nadeem indicated no relevant financial relationships.
Tejas Nikumbh indicated no relevant financial relationships.
Daniel Satterfield indicated no relevant financial relationships.
James Dove indicated no relevant financial relationships.
Craig Wood indicated no relevant financial relationships.
Peter Benotti indicated no relevant financial relationships.
Harshit Khara indicated no relevant financial relationships.
Amitpal Johal indicated no relevant financial relationships.
Christopher Still: Lilly – Consultant, Speakers Bureau. Novo Nordisk – Consultant, Speakers Bureau. Regeneron – Grant/Research Support.
Danial Nadeem, MD1, Tejas Nikumbh, MD2, Daniel Satterfield, CRNP3, James Dove, MS3, Craig Wood, MS3, Peter Benotti, MD3, Harshit S. Khara, MD4, Amitpal Johal, MD3, Christopher Still, DO3. P0853 - Glucagon-Like Peptide-1 Receptor Agonists and Upper Endoscopy: A Data-Driven Approach to Pre-Procedural Optimization, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Geisinger Wyoming Valley Medical Center, Wilkes-Barre, PA; 2The Wright Center for Graduate Medical Education, Dunmore, PA; 3Geisinger Health System, Danville, PA; 4Geisinger Health System, Danville, NJ
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are commonly used in the treatment of type 2 diabetes mellitus and obesity. However, their effect on delaying gastric emptying can complicate upper endoscopy (EGD), where gastric clearance is critical for procedural safety and visualization. Despite these concerns, formal guidance on peri-procedural GLP-1 RA management has been inconsistent. In response, our institution implemented a standardized protocol requiring a 24-hour clear liquid diet and temporary discontinuation of GLP-1 RAs prior to EGD. This study assessed the protocol’s impact on gastric food retention, procedure cancellations, and repeat EGDs within 30 days.
Methods: We conducted a retrospective cohort study across the Geisinger health system comparing outcomes before (January 2022–August 2023) and after (September 2023–December 2024) implementation of a standardized protocol requiring a 24-hour clear liquid diet and temporary discontinuation of GLP-1 RA medications prior to EGD. Daily-dosed medications were held the morning of the procedure, whereas weekly formulations were held for three days prior.
Adults (≥18 years) with GLP-1 RA use within 90 days of outpatient EGD were included; those with gastroparesis were excluded. The primary outcome was gastric food retention per EGD reports. Secondary outcomes included aborted procedures and repeat EGDs within 30 days. Logistic regression was adjusted for age, sex, BMI, and race/ethnicity. Analyses were stratified by diabetes status.
Results: Among 641 pre-intervention and 554 post-intervention EGDs, the mean patient age was 52 years; 69% were female, and 59% had diabetes. Food retention significantly decreased post-intervention (11.4% vs. 5.1%; 95% CI: 0.27–0.65; p = 0.0001) regardless of diabetes status. Among those with food retention, a higher proportion had only small-volume retention post-intervention (30% vs. 4%; p = 0.0043). Repeat EGDs within 30 days declined significantly (2.5% vs. 0.4%; p = 0.0030) and aborted procedures trended lower (1.4% vs. 0.4%; p = 0.072).
Discussion: Implementation of a GLP-1 RA pre-endoscopy guideline significantly reduced food retention, lowering aspiration risk, procedure cancellations, and unnecessary repeat EGDs. These findings highlight a scalable, cost-effective intervention that improves endoscopic efficiency and may reduce healthcare costs by minimizing anesthesia use, provider time, and operating room resources.

Figure: Table 1. Baseline demographic and clinical characteristics of patients undergoing EGD before and after implementation of GLP-1 RA procedural guidance.

Figure: Table 2. Adjusted logistic regression analysis of gastric content retention rates before and after implementation of GLP-1 RA procedural guidance, stratified by diabetes status.
Disclosures:
Danial Nadeem indicated no relevant financial relationships.
Tejas Nikumbh indicated no relevant financial relationships.
Daniel Satterfield indicated no relevant financial relationships.
James Dove indicated no relevant financial relationships.
Craig Wood indicated no relevant financial relationships.
Peter Benotti indicated no relevant financial relationships.
Harshit Khara indicated no relevant financial relationships.
Amitpal Johal indicated no relevant financial relationships.
Christopher Still: Lilly – Consultant, Speakers Bureau. Novo Nordisk – Consultant, Speakers Bureau. Regeneron – Grant/Research Support.
Danial Nadeem, MD1, Tejas Nikumbh, MD2, Daniel Satterfield, CRNP3, James Dove, MS3, Craig Wood, MS3, Peter Benotti, MD3, Harshit S. Khara, MD4, Amitpal Johal, MD3, Christopher Still, DO3. P0853 - Glucagon-Like Peptide-1 Receptor Agonists and Upper Endoscopy: A Data-Driven Approach to Pre-Procedural Optimization, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
