Sunday Poster Session
Category: Functional Bowel Disease
P0821 - Does Rifaximin Treatment Impact Results of Glucose and Lactulose Breath Testing in Patients With Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)?
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Borko Nojkov, MD
Assistant Professor
University of Michigan Medical School
Ann Arbor, MI
Presenting Author(s)
Borko Nojkov, MD1, Matthew Kwok, BS2, Samuel Chey, MPH2, Shanti Eswaran, MD3, Matthew Sturm, MD, PhD1, Prashant Singh, MD1, Allen Lee, MD, MS1, William Chey, MD, FACG4
1University of Michigan Medical School, Ann Arbor, MI; 2University of Michigan, Ann Arbor, MI; 3Michigan Medicine, Ann Arbor, MI; 4University of Michigan Health, Ann Arbor, MI
Introduction: Small intestinal bacterial overgrowth (SIBO) is a condition that symptomatically overlaps with IBS. Clinicians frequently employ glucose or lactulose breath testing (BT) to diagnose or monitor SIBO in IBS patients. However, whether antibiotic therapy for SIBO affects BT results has not been well studied. This study aims to evaluate the diagnostic outcomes of glucose and lactulose BT before and after rifaximin therapy in patients with IBS-D.
Methods: Prospective, tertiary-care center, study of adult patients fulfilling Rome IV diagnostic criteria for IBS-D. Patients with gastrointestinal (e.g. IBD) or systemic (e.g. scleroderma) disease that could explain IBS symptoms were excluded. Other exclusion criteria were: prior GI surgery, recent GI infection/diverticulitis, use of probiotics, and known allergy to glucose or lactulose. Participants completed glucose (75g) and lactulose (25g) BTs (GBT and LBT) on subsequent days per standardized protocol prior to treatment. Subsequently, participants received rifaximin 550mg three times daily x 14 days followed by repeat GBT and LBT 8 weeks after treatment. A positive BT for SIBO was defined as a rise in H2 of ≥20 ppm over baseline within 90 mins of substrate ingestion and/or CH4 ≥10 ppm at any time.
Results: 63 patients completed 238 breath tests in this ongoing study (mean age 42.9 yrs, 61% female, 90% Caucasian, mean BMI 30). The rate of positive GBTs and LBTs at baseline was 28.6% (18/63) and 55.3% (31/56), respectively (p=0.003). The overall proportion of positive tests between baseline and 8 weeks after rifaximin remained similar for both GBT and LBT (Table 1). However, of the patients who tested positive at baseline, 33% (6/18; GBT) and 48% (15/31; LBT) converted to negative post rifaximin. Inversely, 13.3% (6/45) and 25% (8/32) of patients who tested negative at baseline converted to positive post rifaximin for GBT and LBT, respectively (Table 2). Baseline BT results remained unchanged post rifaximin in significantly more patients with GBT compared to LBT (Table 2). Overall, there were no significant differences in the average breath hydrogen and methane concentrations at baseline and after ingestion of glucose or lactulose before and after rifaximin.
Discussion: There are substantial differences in the rate of positive glucose and lactulose BTs in patients with IBS-D. Although most BTs remain unchanged post rifaximin, around a third of positive GBT and up to half of positive LBT results will convert to negative 8 weeks after rifaximin.
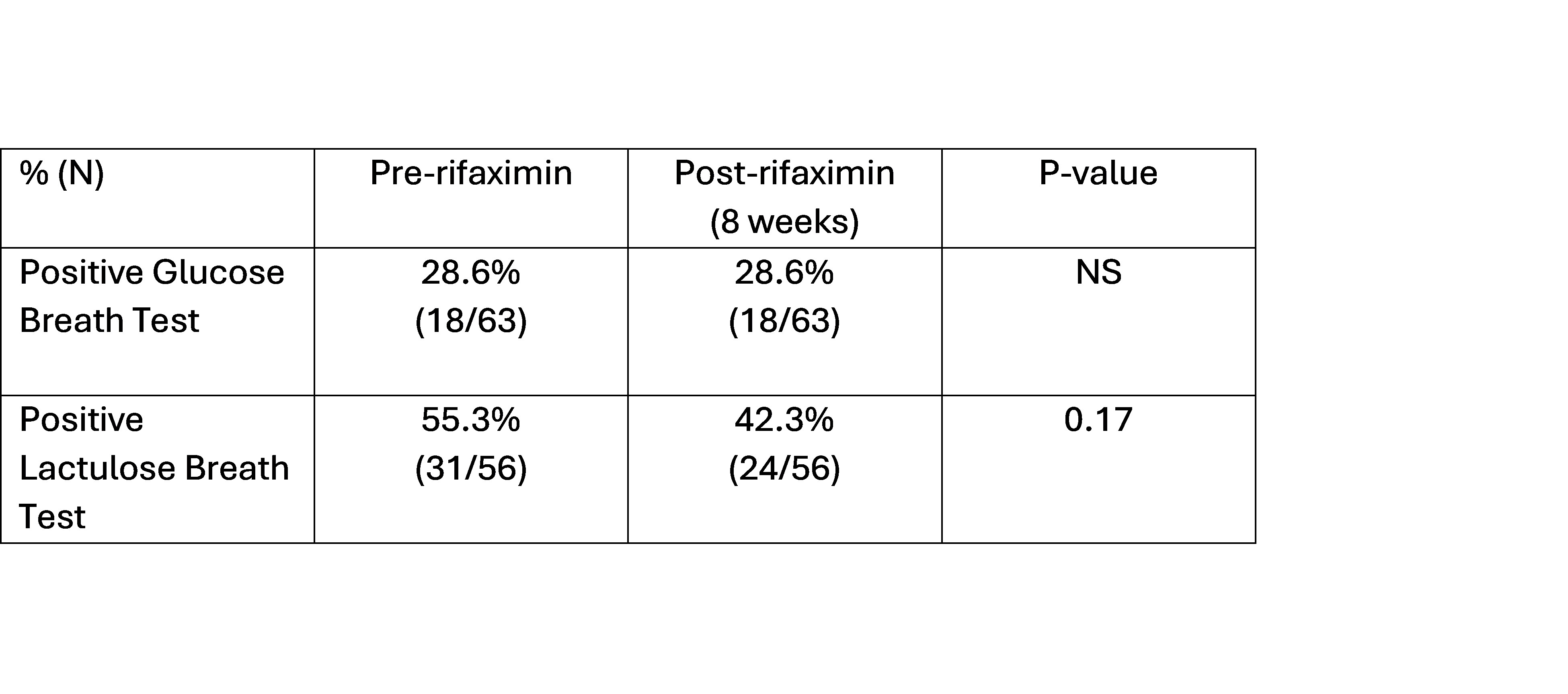
Figure: Proportions of positive glucose and lactulose breath tests before and after rifaximin therapy.
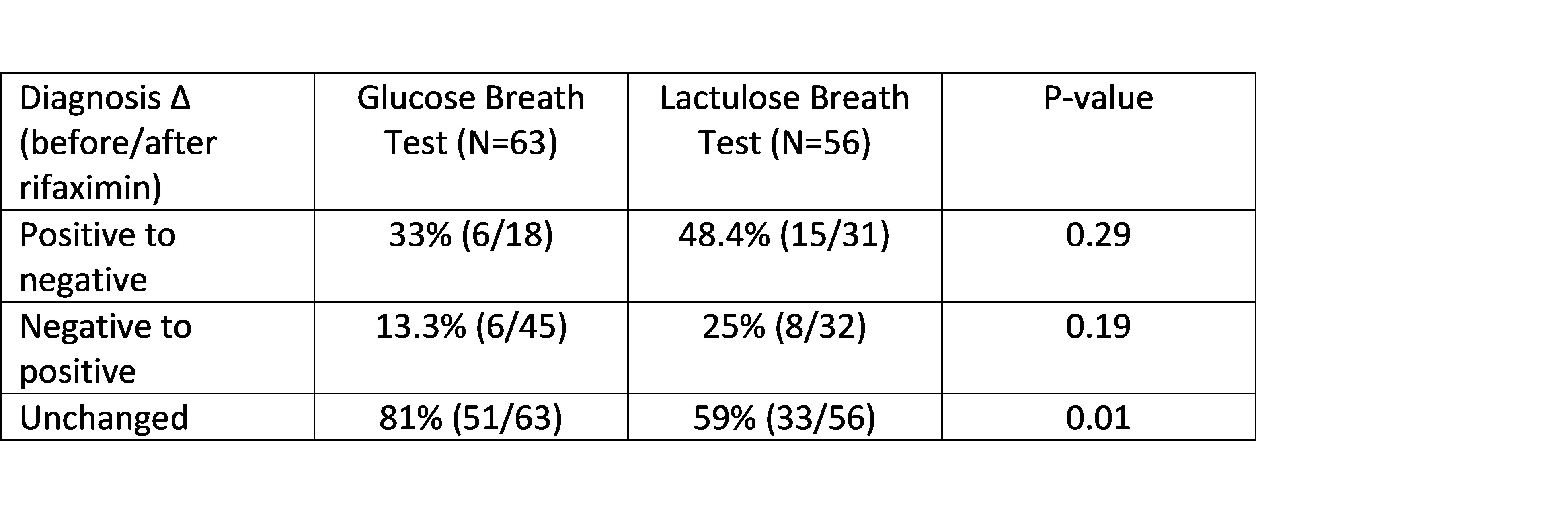
Figure: Diagnostic change of glucose and lactulose breath test results between baseline (pre-rifaximin) and post-rifaximin therapy.
Disclosures:
Borko Nojkov indicated no relevant financial relationships.
Matthew Kwok indicated no relevant financial relationships.
Samuel Chey indicated no relevant financial relationships.
Shanti Eswaran indicated no relevant financial relationships.
Matthew Sturm indicated no relevant financial relationships.
Prashant Singh: Uptodate – Royalties.
Allen Lee: Atmo Biosciences – Consultant.
William Chey: Ardelyx – Consultant. Atmo – Consultant. Biomerica – Consultant. Commonwealth Diagnostics International – Grant/Research Support. Coprata – Stock Options. Dieta – Stock Options. Digital Manometry – Intellectual Property/Patents. Evinature – Stock Options. FoodMarble – Stock Options. Gemelli – Consultant. Kiwi BioScience – Stock Options. Modify Health – Stock Options. My Nutrition Health – Intellectual Property/Patents. Phathom – Consultant. Rectal Expulsion Device – Intellectual Property/Patents. Redhill – Consultant. Salix – Consultant, Grant/Research Support. Takeda – Consultant. Vibrant – Consultant.
Borko Nojkov, MD1, Matthew Kwok, BS2, Samuel Chey, MPH2, Shanti Eswaran, MD3, Matthew Sturm, MD, PhD1, Prashant Singh, MD1, Allen Lee, MD, MS1, William Chey, MD, FACG4. P0821 - Does Rifaximin Treatment Impact Results of Glucose and Lactulose Breath Testing in Patients With Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)?, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Michigan Medical School, Ann Arbor, MI; 2University of Michigan, Ann Arbor, MI; 3Michigan Medicine, Ann Arbor, MI; 4University of Michigan Health, Ann Arbor, MI
Introduction: Small intestinal bacterial overgrowth (SIBO) is a condition that symptomatically overlaps with IBS. Clinicians frequently employ glucose or lactulose breath testing (BT) to diagnose or monitor SIBO in IBS patients. However, whether antibiotic therapy for SIBO affects BT results has not been well studied. This study aims to evaluate the diagnostic outcomes of glucose and lactulose BT before and after rifaximin therapy in patients with IBS-D.
Methods: Prospective, tertiary-care center, study of adult patients fulfilling Rome IV diagnostic criteria for IBS-D. Patients with gastrointestinal (e.g. IBD) or systemic (e.g. scleroderma) disease that could explain IBS symptoms were excluded. Other exclusion criteria were: prior GI surgery, recent GI infection/diverticulitis, use of probiotics, and known allergy to glucose or lactulose. Participants completed glucose (75g) and lactulose (25g) BTs (GBT and LBT) on subsequent days per standardized protocol prior to treatment. Subsequently, participants received rifaximin 550mg three times daily x 14 days followed by repeat GBT and LBT 8 weeks after treatment. A positive BT for SIBO was defined as a rise in H2 of ≥20 ppm over baseline within 90 mins of substrate ingestion and/or CH4 ≥10 ppm at any time.
Results: 63 patients completed 238 breath tests in this ongoing study (mean age 42.9 yrs, 61% female, 90% Caucasian, mean BMI 30). The rate of positive GBTs and LBTs at baseline was 28.6% (18/63) and 55.3% (31/56), respectively (p=0.003). The overall proportion of positive tests between baseline and 8 weeks after rifaximin remained similar for both GBT and LBT (Table 1). However, of the patients who tested positive at baseline, 33% (6/18; GBT) and 48% (15/31; LBT) converted to negative post rifaximin. Inversely, 13.3% (6/45) and 25% (8/32) of patients who tested negative at baseline converted to positive post rifaximin for GBT and LBT, respectively (Table 2). Baseline BT results remained unchanged post rifaximin in significantly more patients with GBT compared to LBT (Table 2). Overall, there were no significant differences in the average breath hydrogen and methane concentrations at baseline and after ingestion of glucose or lactulose before and after rifaximin.
Discussion: There are substantial differences in the rate of positive glucose and lactulose BTs in patients with IBS-D. Although most BTs remain unchanged post rifaximin, around a third of positive GBT and up to half of positive LBT results will convert to negative 8 weeks after rifaximin.

Figure: Proportions of positive glucose and lactulose breath tests before and after rifaximin therapy.

Figure: Diagnostic change of glucose and lactulose breath test results between baseline (pre-rifaximin) and post-rifaximin therapy.
Disclosures:
Borko Nojkov indicated no relevant financial relationships.
Matthew Kwok indicated no relevant financial relationships.
Samuel Chey indicated no relevant financial relationships.
Shanti Eswaran indicated no relevant financial relationships.
Matthew Sturm indicated no relevant financial relationships.
Prashant Singh: Uptodate – Royalties.
Allen Lee: Atmo Biosciences – Consultant.
William Chey: Ardelyx – Consultant. Atmo – Consultant. Biomerica – Consultant. Commonwealth Diagnostics International – Grant/Research Support. Coprata – Stock Options. Dieta – Stock Options. Digital Manometry – Intellectual Property/Patents. Evinature – Stock Options. FoodMarble – Stock Options. Gemelli – Consultant. Kiwi BioScience – Stock Options. Modify Health – Stock Options. My Nutrition Health – Intellectual Property/Patents. Phathom – Consultant. Rectal Expulsion Device – Intellectual Property/Patents. Redhill – Consultant. Salix – Consultant, Grant/Research Support. Takeda – Consultant. Vibrant – Consultant.
Borko Nojkov, MD1, Matthew Kwok, BS2, Samuel Chey, MPH2, Shanti Eswaran, MD3, Matthew Sturm, MD, PhD1, Prashant Singh, MD1, Allen Lee, MD, MS1, William Chey, MD, FACG4. P0821 - Does Rifaximin Treatment Impact Results of Glucose and Lactulose Breath Testing in Patients With Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)?, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
