Sunday Poster Session
Category: Esophagus
P0669 - Effect of Social Determinants of Health on Healthcare Costs and Access to Care in Patients With Eosinophilic Esophagitis: A Retrospective Cohort Study of US Health Insurance Claims Data
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- BG
Bridgett Goodwin, PhD
Takeda Pharmaceuticals USA, Inc.
Lexington, MA
Presenting Author(s)
Elizabeth T. Jensen, PhD1, Benjamin D. Gold, MD2, Bridgett Goodwin, PhD3, Yiyan Liu, PhD3, Michael Kim, PharmD4, Taylor T. Schwartz, MPH5, Carolyn R. Schaeffer-Koziol, PhD3, Brian Terreri, PharmD3, Alan P. Baptist, MD, MPH6
1Wake Forest University School of Medicine, Winston-Salem, NC; 2GI Care for Kids, Children’s Center for Digestive Healthcare, Atlanta, GA; 3Takeda Pharmaceuticals USA, Inc., Lexington, MA; 4Takeda Pharmaceuticals USA, Inc., Chicago, IL; 5Avalere Health, Washington, DC; 6Division of Allergy and Clinical Immunology, Henry Ford Health and Michigan State University Health Sciences, Detroit, MI
Introduction: Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease characterized by symptoms of esophageal dysfunction. Social determinants of health (SDoH) may affect clinical outcomes.
Methods: This retrospective, longitudinal cohort study (January 1, 2016–December 31, 2022) examined US health insurance claims and enrollment data for patients with EoE from the Inovalon closed claims (ICC) database and the 100% sample of Medicare Fee-For-Service (MFFS) parts A/B/D. Eligible patients had ≥2 claims (≥30 days apart) with a diagnosis code for EoE (ICD-10-CM: K20.0) in the index period (January 1, 2017–December 31, 2021 [index date=first of these claims]); had continuous enrollment in medical and pharmacy benefits for ≥12 months before and after the index date (baseline and follow-up periods, respectively); and were aged ≥11 years at index. Patients were excluded if they had a claim for eosinophilic gastritis/gastroenteritis (ICD-10-CM: K52.81) post-index. SDoH data were linked via Zip code to patients from the Acxiom Market Indices and other sources using a HIPAA-compliant safe harbor database. The effect of SDoH factors (Area Deprivation Index [ADI], distance to a patient’s diagnosing provider and rurality at index) on EoE-related healthcare costs, time to diagnosis and likelihood of pharmacological treatment was examined during follow-up (except for time to diagnosis, which was assessed from disease onset [baseline] to diagnosis [index date]) using regression modelling.
Results: Overall, 40,619 patients diagnosed with EoE were included (ICC, n=26,340; MFFS, n=14,279). Patient demographics (ICC and MFFS): mean (SD) age at index, 38.0 (16.8) and 67.0 (13.1) years; male, 58.8% and 50.4%. For both ICC and MFFS, patients diagnosed with EoE living in urban/suburban areas were diagnosed significantly more quickly than those living in rural areas (ICC, by 36.5 days, p< 0.001; MFFS, by 32.9 days, p=0.02 (Table 1). Patients living in Zip codes in the 4th−5th (i.e. most deprived) versus the 1st−3rd quintiles for the ADI had a 6% increase in costs (p< 0.001; ICC only), had a longer time to diagnosis (by 32.9 days, p< 0.001; ICC only) and were more likely to receive treatment (ICC, by 18%, p< 0.001; MFFS, by 23%, p=0.003 (Table 1).
Discussion: SDoH can affect time to diagnosis, healthcare costs and the likelihood of treatment for patients with EoE; these results, along with the effect of sex, race and how ADI and rurality interact, require further investigation.
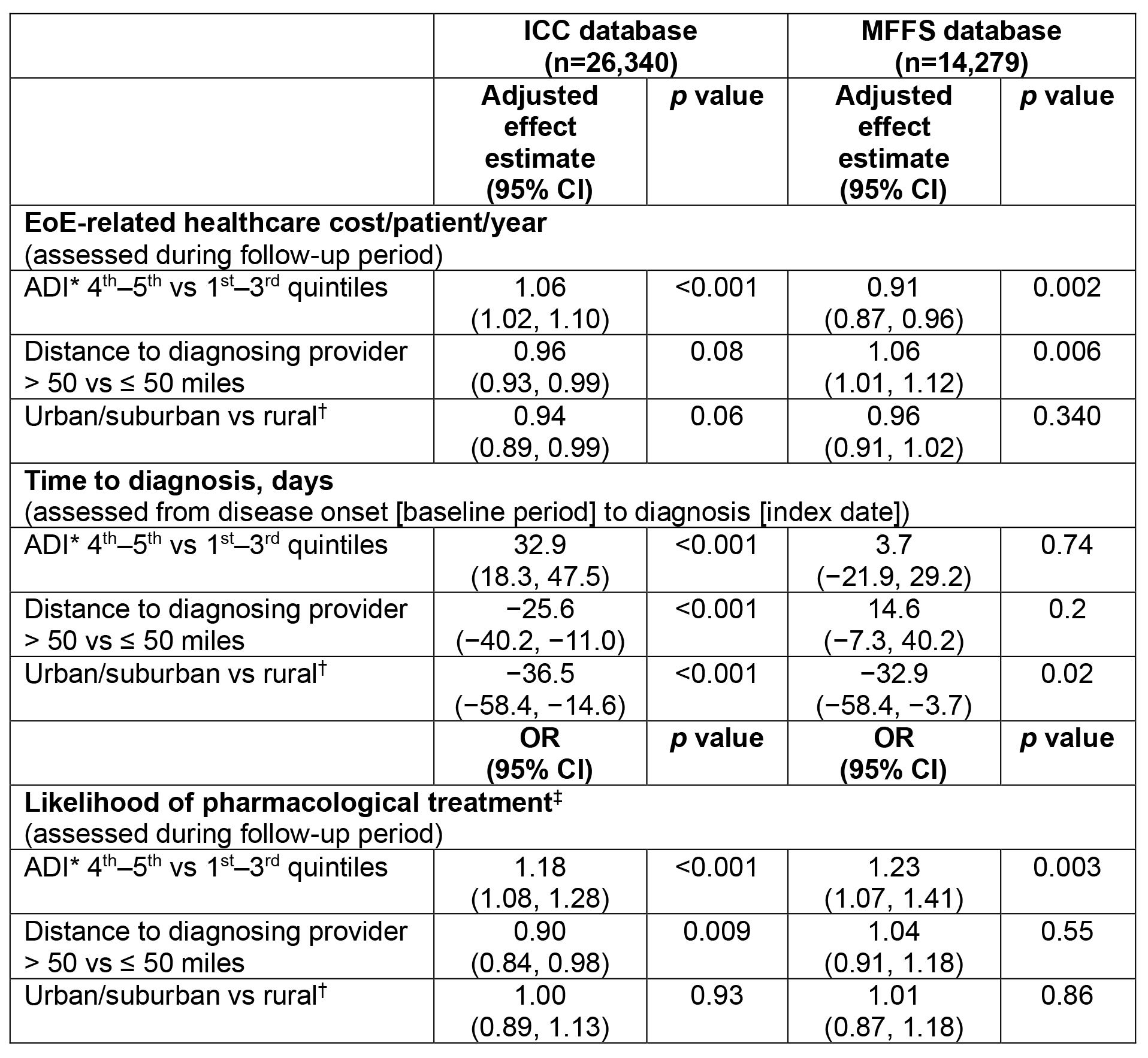
Figure: Table 1. Effect of SDoH factors on EoE-related healthcare costs, time to diagnosis and likelihood of treatment in patients diagnosed with EoE in the ICC and MFFS databases.
General linearized regression modelling with log-transformed costs was used for the EoE-related healthcare cost analysis; linear regression modelling was used for the time-to-diagnosis analysis; and logistic regression modelling was used for the likelihood of treatment analysis. Models were adjusted for potentially confounding factors, including the following covariates: centered age (38.11 years), sex, census region, race/ethnicity (MFFS database only), low-income subsidy (MFFS database only), time between onset of symptoms and diagnosis (EoE-related healthcare cost [both databases] and likelihood of treatment [ICC database only]), weighted Charlson Comorbidity Index and the SDoH factors.
*Measures the socioeconomic condition of a region based on publicly available data in the domains of income, education, employment and housing quality; rankings range from 0 to 100 (0 = least disadvantaged; 100 = most disadvantaged); therefore, the 4th and 5th quintiles represent more deprived regions than the 1st to 3rd quintiles.
†Rural includes large rural towns, small towns, isolated rural and other rural.
‡Treatment likelihood was modelled as a binary (yes/no) outcome, with ‘yes’ defined as having ≥1 claim for a treatment of interest (budesonide oral suspension, systemic or topical corticosteroids, dupilumab, other biologic therapies, or proton-pump inhibitors).
ADI, Area Deprivation Index; CI, confidence interval; EoE, eosinophilic esophagitis; ICC, Inovalon closed claims; MFFS, Medicare Fee-For-Service; OR, odds ratio; SDoH, social determinants of health.
Disclosures:
Elizabeth Jensen: Jazz Pharmaceuticals – Consultant. Regeneron/Sanofi – Advisory Committee/Board Member, Consultant, Grant/Research Support. Takeda – Consultant. Target RWD/RWE – Consultant.
Benjamin Gold: DiaSorin Molecular – Consultant, participates in continuing medical education activities. Ironwood Pharmaceuticals – Consultant, participates in continuing medical education activities. Johnson & Johnson (Janssen) – Consultant, participates in continuing medical education activities. Mead Johnson Nutrition – Consultant, participates in continuing medical education activities. Nutricia North America – Consultant, participates in continuing medical education activities. Takeda Pharmaceutical Company Limited – Consultant, participates in continuing medical education activities.
Bridgett Goodwin: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Yiyan Liu: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Michael Kim: Takeda Pharmaceuticals USA, Inc. – Employee.
Taylor Schwartz: Avalere – Employee, Avalere was funded by Takeda Pharmaceuticals USA, Inc. to conduct the study.
Carolyn Schaeffer-Koziol: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Brian Terreri: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Alan Baptist: AstraZeneca – Grant/Research Support. GSK – Consultant, Grant/Research Support. Novartis – Grant/Research Support. Takeda – Grant/Research Support. Teva Pharmaceuticals – Consultant.
Elizabeth T. Jensen, PhD1, Benjamin D. Gold, MD2, Bridgett Goodwin, PhD3, Yiyan Liu, PhD3, Michael Kim, PharmD4, Taylor T. Schwartz, MPH5, Carolyn R. Schaeffer-Koziol, PhD3, Brian Terreri, PharmD3, Alan P. Baptist, MD, MPH6. P0669 - Effect of Social Determinants of Health on Healthcare Costs and Access to Care in Patients With Eosinophilic Esophagitis: A Retrospective Cohort Study of US Health Insurance Claims Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Wake Forest University School of Medicine, Winston-Salem, NC; 2GI Care for Kids, Children’s Center for Digestive Healthcare, Atlanta, GA; 3Takeda Pharmaceuticals USA, Inc., Lexington, MA; 4Takeda Pharmaceuticals USA, Inc., Chicago, IL; 5Avalere Health, Washington, DC; 6Division of Allergy and Clinical Immunology, Henry Ford Health and Michigan State University Health Sciences, Detroit, MI
Introduction: Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease characterized by symptoms of esophageal dysfunction. Social determinants of health (SDoH) may affect clinical outcomes.
Methods: This retrospective, longitudinal cohort study (January 1, 2016–December 31, 2022) examined US health insurance claims and enrollment data for patients with EoE from the Inovalon closed claims (ICC) database and the 100% sample of Medicare Fee-For-Service (MFFS) parts A/B/D. Eligible patients had ≥2 claims (≥30 days apart) with a diagnosis code for EoE (ICD-10-CM: K20.0) in the index period (January 1, 2017–December 31, 2021 [index date=first of these claims]); had continuous enrollment in medical and pharmacy benefits for ≥12 months before and after the index date (baseline and follow-up periods, respectively); and were aged ≥11 years at index. Patients were excluded if they had a claim for eosinophilic gastritis/gastroenteritis (ICD-10-CM: K52.81) post-index. SDoH data were linked via Zip code to patients from the Acxiom Market Indices and other sources using a HIPAA-compliant safe harbor database. The effect of SDoH factors (Area Deprivation Index [ADI], distance to a patient’s diagnosing provider and rurality at index) on EoE-related healthcare costs, time to diagnosis and likelihood of pharmacological treatment was examined during follow-up (except for time to diagnosis, which was assessed from disease onset [baseline] to diagnosis [index date]) using regression modelling.
Results: Overall, 40,619 patients diagnosed with EoE were included (ICC, n=26,340; MFFS, n=14,279). Patient demographics (ICC and MFFS): mean (SD) age at index, 38.0 (16.8) and 67.0 (13.1) years; male, 58.8% and 50.4%. For both ICC and MFFS, patients diagnosed with EoE living in urban/suburban areas were diagnosed significantly more quickly than those living in rural areas (ICC, by 36.5 days, p< 0.001; MFFS, by 32.9 days, p=0.02 (Table 1). Patients living in Zip codes in the 4th−5th (i.e. most deprived) versus the 1st−3rd quintiles for the ADI had a 6% increase in costs (p< 0.001; ICC only), had a longer time to diagnosis (by 32.9 days, p< 0.001; ICC only) and were more likely to receive treatment (ICC, by 18%, p< 0.001; MFFS, by 23%, p=0.003 (Table 1).
Discussion: SDoH can affect time to diagnosis, healthcare costs and the likelihood of treatment for patients with EoE; these results, along with the effect of sex, race and how ADI and rurality interact, require further investigation.

Figure: Table 1. Effect of SDoH factors on EoE-related healthcare costs, time to diagnosis and likelihood of treatment in patients diagnosed with EoE in the ICC and MFFS databases.
General linearized regression modelling with log-transformed costs was used for the EoE-related healthcare cost analysis; linear regression modelling was used for the time-to-diagnosis analysis; and logistic regression modelling was used for the likelihood of treatment analysis. Models were adjusted for potentially confounding factors, including the following covariates: centered age (38.11 years), sex, census region, race/ethnicity (MFFS database only), low-income subsidy (MFFS database only), time between onset of symptoms and diagnosis (EoE-related healthcare cost [both databases] and likelihood of treatment [ICC database only]), weighted Charlson Comorbidity Index and the SDoH factors.
*Measures the socioeconomic condition of a region based on publicly available data in the domains of income, education, employment and housing quality; rankings range from 0 to 100 (0 = least disadvantaged; 100 = most disadvantaged); therefore, the 4th and 5th quintiles represent more deprived regions than the 1st to 3rd quintiles.
†Rural includes large rural towns, small towns, isolated rural and other rural.
‡Treatment likelihood was modelled as a binary (yes/no) outcome, with ‘yes’ defined as having ≥1 claim for a treatment of interest (budesonide oral suspension, systemic or topical corticosteroids, dupilumab, other biologic therapies, or proton-pump inhibitors).
ADI, Area Deprivation Index; CI, confidence interval; EoE, eosinophilic esophagitis; ICC, Inovalon closed claims; MFFS, Medicare Fee-For-Service; OR, odds ratio; SDoH, social determinants of health.
Disclosures:
Elizabeth Jensen: Jazz Pharmaceuticals – Consultant. Regeneron/Sanofi – Advisory Committee/Board Member, Consultant, Grant/Research Support. Takeda – Consultant. Target RWD/RWE – Consultant.
Benjamin Gold: DiaSorin Molecular – Consultant, participates in continuing medical education activities. Ironwood Pharmaceuticals – Consultant, participates in continuing medical education activities. Johnson & Johnson (Janssen) – Consultant, participates in continuing medical education activities. Mead Johnson Nutrition – Consultant, participates in continuing medical education activities. Nutricia North America – Consultant, participates in continuing medical education activities. Takeda Pharmaceutical Company Limited – Consultant, participates in continuing medical education activities.
Bridgett Goodwin: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Yiyan Liu: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Michael Kim: Takeda Pharmaceuticals USA, Inc. – Employee.
Taylor Schwartz: Avalere – Employee, Avalere was funded by Takeda Pharmaceuticals USA, Inc. to conduct the study.
Carolyn Schaeffer-Koziol: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Brian Terreri: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Alan Baptist: AstraZeneca – Grant/Research Support. GSK – Consultant, Grant/Research Support. Novartis – Grant/Research Support. Takeda – Grant/Research Support. Teva Pharmaceuticals – Consultant.
Elizabeth T. Jensen, PhD1, Benjamin D. Gold, MD2, Bridgett Goodwin, PhD3, Yiyan Liu, PhD3, Michael Kim, PharmD4, Taylor T. Schwartz, MPH5, Carolyn R. Schaeffer-Koziol, PhD3, Brian Terreri, PharmD3, Alan P. Baptist, MD, MPH6. P0669 - Effect of Social Determinants of Health on Healthcare Costs and Access to Care in Patients With Eosinophilic Esophagitis: A Retrospective Cohort Study of US Health Insurance Claims Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
