Sunday Poster Session
Category: Esophagus
P0653 - Artificial Intelligence-Based Radiomic Prediction of Neoadjuvant Chemoradiotherapy Treatment Response in Esophageal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Mark Nasseem, MBBCh (he/him/his)
Ain Shams University
Presenting Author(s)
Zainab Hussein, 1, Mohamed A. B. Elnaggar, MD2, Omar F. Abbas, 3, Ismail Elkhattib, MBBCh4, Mark Nasseem, MBBCh5, Basant Elsayed, MBBCh6, Ahmed Bahnasy, MD7, Jubran K. Alzedaar, 8, Timothy S. Boyd, MD9
1Faculty of Medicine, Minia University, Minia, Al Minya, Egypt; 2Hartford Healthcare, Hartford, CT; 3Al-Azhar University, Cairo, Al Qahirah, Egypt; 4University of Nebraska, Hartford, CT; 5Ain Shams University, Cairo, Al Qahirah, Egypt; 6Ain Shams University, Cairo, Ad Daqahliyah, Egypt; 7Mayo Clinic, Rochester, MN; 821 September University for medical and applied sciences, Sanaa, San'a', Yemen; 9Hartford HealthCare, Hartford, CT
Introduction: Accurate prediction of pathological response to neoadjuvant chemoradiotherapy (nCRT) in esophageal squamous cell carcinoma (ESCC) remains a major clinical challenge. While current predictors are inadequate, AI-driven radiomics offers a transformative approach. This meta-analysis evaluates the performance of AI-based radiomics for predicting nCRT response, which directly addresses the need for personalized therapeutic strategies.
Methods: We conducted a PRISMA-compliant systematic review, searching PubMed, Embase, Scopus, and Cochrane through March 2025. Original studies developing and testing AI-radiomic models (machine/deep learning) to predict pathological response in ESCC post-nCRT were included. Methodological quality was assessed via QUADAS-2. Bivariate random-effects models pooled sensitivity, specificity, and AUC (95% CI), with heterogeneity quantified using I² statistics. PROSPERO ID: (CRD420251024295)
Results: Ten studies and 1,519 patients were included in our analysis. Radiomics achieved the following diagnostic test measures: (sensitivity: 0.804, 95% CI[ 0.75–0.85]; I² = 66.5%, p < 0.001), (specificity: 0.816, 95% CI [0.78–0.85]; I² = 51.0%, p < 0.001), and (AUC: 0.85, 95% CI [0.81–0.88]; I² = 86.7%, p < 0.0001). Heterogeneity reflected variations in radiomic feature extraction but was balanced by consistent endpoint assessment (Mandard TRG 1–2). Eighty percent of the included studies showed low bias risk.
Discussion: AI-based radiomics demonstrates high discriminatory power (AUC 0.85) for stratifying ESCC patients by nCRT response likelihood. This supports its clinical utility in guiding treatment intensification in predicted poor responders, enabling personalized de-escalation (e.g., organ preservation) in predicted responders, and potentially reducing unnecessary surgery. Prospective multicenter validation and standardized radiomic pipelines are imperative for clinical adoption
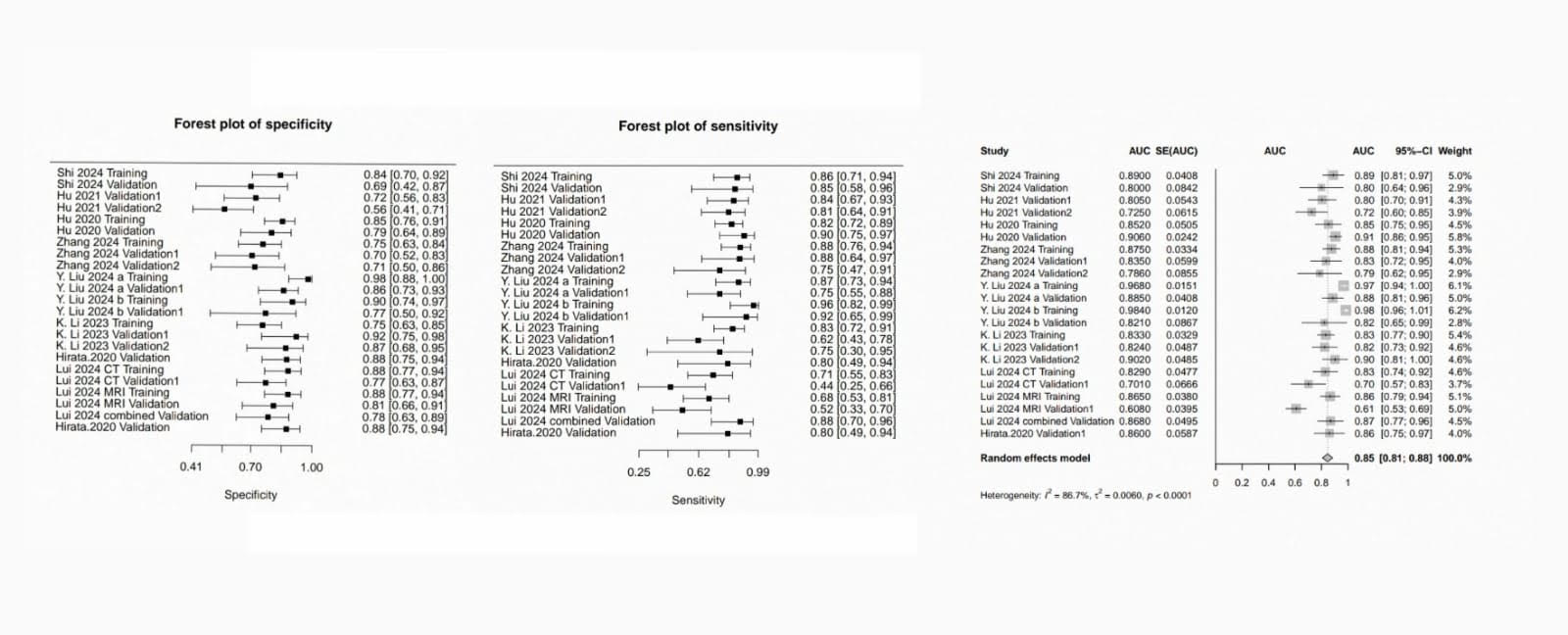
Figure: Bivariate random-effects models pooled AUC (95% CI), sensitivity and specificity with heterogeneity quantified using I² statistics.
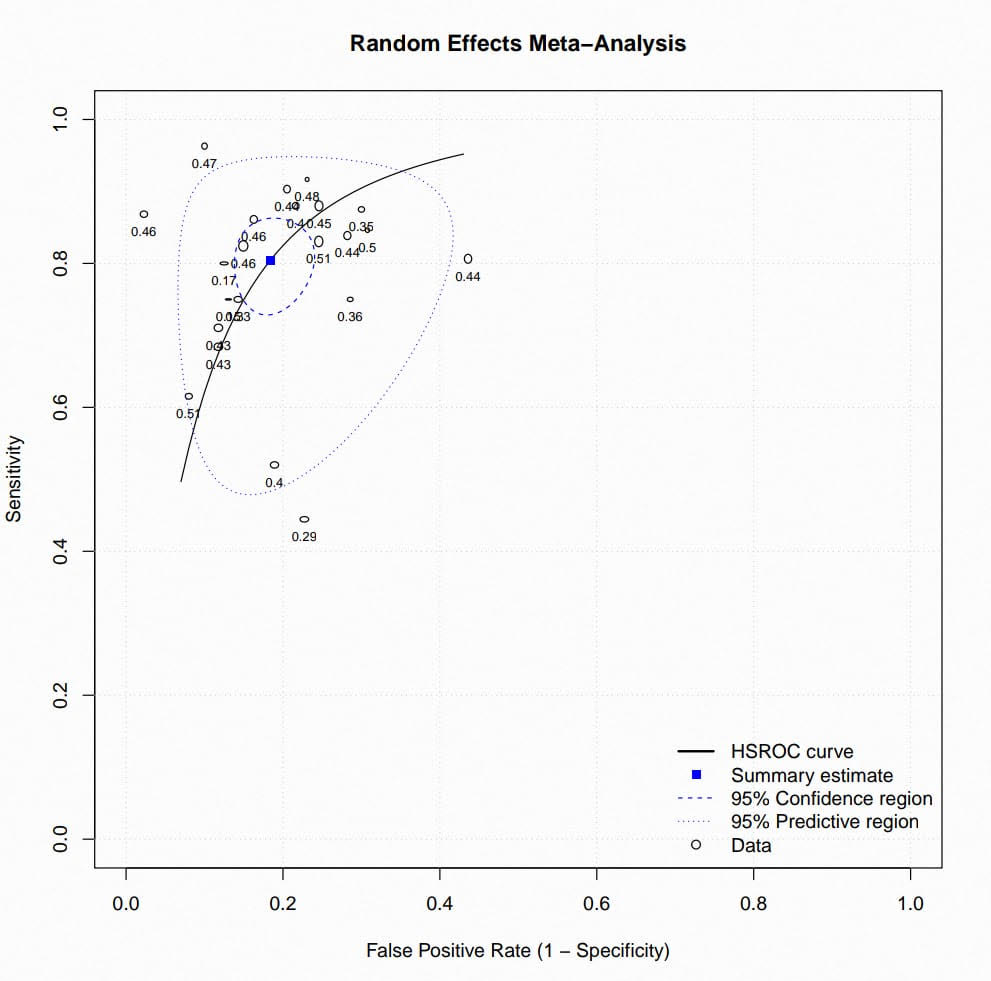
Figure: Random effects meta-analysis , HSROC curve
Disclosures:
Zainab Hussein indicated no relevant financial relationships.
Mohamed A. Elnaggar indicated no relevant financial relationships.
Omar F. Abbas indicated no relevant financial relationships.
Ismail Elkhattib indicated no relevant financial relationships.
Mark Nasseem indicated no relevant financial relationships.
Basant Elsayed indicated no relevant financial relationships.
Ahmed Bahnasy indicated no relevant financial relationships.
Jubran K. Alzedaar indicated no relevant financial relationships.
Timothy Boyd indicated no relevant financial relationships.
Zainab Hussein, 1, Mohamed A. B. Elnaggar, MD2, Omar F. Abbas, 3, Ismail Elkhattib, MBBCh4, Mark Nasseem, MBBCh5, Basant Elsayed, MBBCh6, Ahmed Bahnasy, MD7, Jubran K. Alzedaar, 8, Timothy S. Boyd, MD9. P0653 - Artificial Intelligence-Based Radiomic Prediction of Neoadjuvant Chemoradiotherapy Treatment Response in Esophageal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Faculty of Medicine, Minia University, Minia, Al Minya, Egypt; 2Hartford Healthcare, Hartford, CT; 3Al-Azhar University, Cairo, Al Qahirah, Egypt; 4University of Nebraska, Hartford, CT; 5Ain Shams University, Cairo, Al Qahirah, Egypt; 6Ain Shams University, Cairo, Ad Daqahliyah, Egypt; 7Mayo Clinic, Rochester, MN; 821 September University for medical and applied sciences, Sanaa, San'a', Yemen; 9Hartford HealthCare, Hartford, CT
Introduction: Accurate prediction of pathological response to neoadjuvant chemoradiotherapy (nCRT) in esophageal squamous cell carcinoma (ESCC) remains a major clinical challenge. While current predictors are inadequate, AI-driven radiomics offers a transformative approach. This meta-analysis evaluates the performance of AI-based radiomics for predicting nCRT response, which directly addresses the need for personalized therapeutic strategies.
Methods: We conducted a PRISMA-compliant systematic review, searching PubMed, Embase, Scopus, and Cochrane through March 2025. Original studies developing and testing AI-radiomic models (machine/deep learning) to predict pathological response in ESCC post-nCRT were included. Methodological quality was assessed via QUADAS-2. Bivariate random-effects models pooled sensitivity, specificity, and AUC (95% CI), with heterogeneity quantified using I² statistics. PROSPERO ID: (CRD420251024295)
Results: Ten studies and 1,519 patients were included in our analysis. Radiomics achieved the following diagnostic test measures: (sensitivity: 0.804, 95% CI[ 0.75–0.85]; I² = 66.5%, p < 0.001), (specificity: 0.816, 95% CI [0.78–0.85]; I² = 51.0%, p < 0.001), and (AUC: 0.85, 95% CI [0.81–0.88]; I² = 86.7%, p < 0.0001). Heterogeneity reflected variations in radiomic feature extraction but was balanced by consistent endpoint assessment (Mandard TRG 1–2). Eighty percent of the included studies showed low bias risk.
Discussion: AI-based radiomics demonstrates high discriminatory power (AUC 0.85) for stratifying ESCC patients by nCRT response likelihood. This supports its clinical utility in guiding treatment intensification in predicted poor responders, enabling personalized de-escalation (e.g., organ preservation) in predicted responders, and potentially reducing unnecessary surgery. Prospective multicenter validation and standardized radiomic pipelines are imperative for clinical adoption

Figure: Bivariate random-effects models pooled AUC (95% CI), sensitivity and specificity with heterogeneity quantified using I² statistics.

Figure: Random effects meta-analysis , HSROC curve
Disclosures:
Zainab Hussein indicated no relevant financial relationships.
Mohamed A. Elnaggar indicated no relevant financial relationships.
Omar F. Abbas indicated no relevant financial relationships.
Ismail Elkhattib indicated no relevant financial relationships.
Mark Nasseem indicated no relevant financial relationships.
Basant Elsayed indicated no relevant financial relationships.
Ahmed Bahnasy indicated no relevant financial relationships.
Jubran K. Alzedaar indicated no relevant financial relationships.
Timothy Boyd indicated no relevant financial relationships.
Zainab Hussein, 1, Mohamed A. B. Elnaggar, MD2, Omar F. Abbas, 3, Ismail Elkhattib, MBBCh4, Mark Nasseem, MBBCh5, Basant Elsayed, MBBCh6, Ahmed Bahnasy, MD7, Jubran K. Alzedaar, 8, Timothy S. Boyd, MD9. P0653 - Artificial Intelligence-Based Radiomic Prediction of Neoadjuvant Chemoradiotherapy Treatment Response in Esophageal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
