Sunday Poster Session
Category: Esophagus
P0647 - De-Escalating Proton Pump Inhibitor Use in Outpatient Setting – A Quality Improvement Venture
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- WK
Warsha Mal Korani, MD
Conemaugh Memorial Medical Center
Johnstown, PA
Presenting Author(s)
Warsha Korani, MD1, Sanjana Kamat, 2, Darpan Sohni, 2, Raaghvi Kohli, MD3, Laiba Khan, 2, Euzebio Ribeiro Silva, 2, Saba Waseem, 2
1Conemaugh Memorial Medical Center, Johnstown, PA; 2Conemaugh memorial medical center, Johnstown, PA; 3Conemaugh Memorial Medical Centre, Johnstown, PA
Introduction:
Proton pump inhibitors (PPIs) are among the most commonly used medications for acid-related gastrointestinal diseases, including GERD, PUD, Barrett’s esophagus, and Zollinger-Ellison syndrome. However, chronic use is associated with increased risks of fractures, chronic kidney disease, Clostridioides difficile infection, hypomagnesemia, and vitamin B12 deficiency. Despite guideline recommendations from the American Gastroenterological Association (AGA) and American College of Gastroenterology (ACG) for regular review and deprescription, many patients continue PPI therapy indefinitely without reassessment or appropriate endoscopic evaluation.
Methods: Patients aged ≥18 years on long-term PPI therapy were identified during routine clinic visits. Exclusion criteria included Barrett’s esophagus, peptic ulcer disease, upper gastrointestinal bleeding, or chronic high-dose NSAID use. Standardized educational materials were provided to patients outlining the risks of chronic PPI use. Deprescribing strategies included dose down-titration, transition to H2 receptor antagonists, or as-needed therapy. Outcomes assessed included symptom control using the GERD-Q questionnaire, rescue medication use, compliance with deprescribing, and prior history of endoscopic evaluation.
Results: A total of 29 patients were reviewed. The median PPI duration was 4 years. Despite long-term therapy, only 13 patients (44.8%) had previously undergone esophagogastroduodenoscopy (EGD), falling short of ACG guidelines recommending endoscopy after 8 weeks of refractory symptoms. Fourteen patients (48.3%) successfully complied with deprescribing. Three patients (10.3%) refused, and two (6.9%) were unreachable for follow-up. At follow-up, 50% had good symptom control (GERD-Q ≤7), 16.7% had borderline symptoms (8–10), and 25% had significant symptoms (≥11). Rescue medication use was reported in 41.7% of cases, with nausea being the most common post-deprescribing symptom. No serious adverse events occurred.
Discussion: This preliminary analysis highlights that nearly half of patients could be successfully deprescribed, with no major adverse events. The majority had been on PPIs for years without appropriate endoscopic evaluation. As additional patients are followed in the coming weeks, this project underscores the urgent need to integrate deprescribing protocols, enhance physician awareness, and engage patients through education in order to optimize the safe use of PPIs in outpatient care
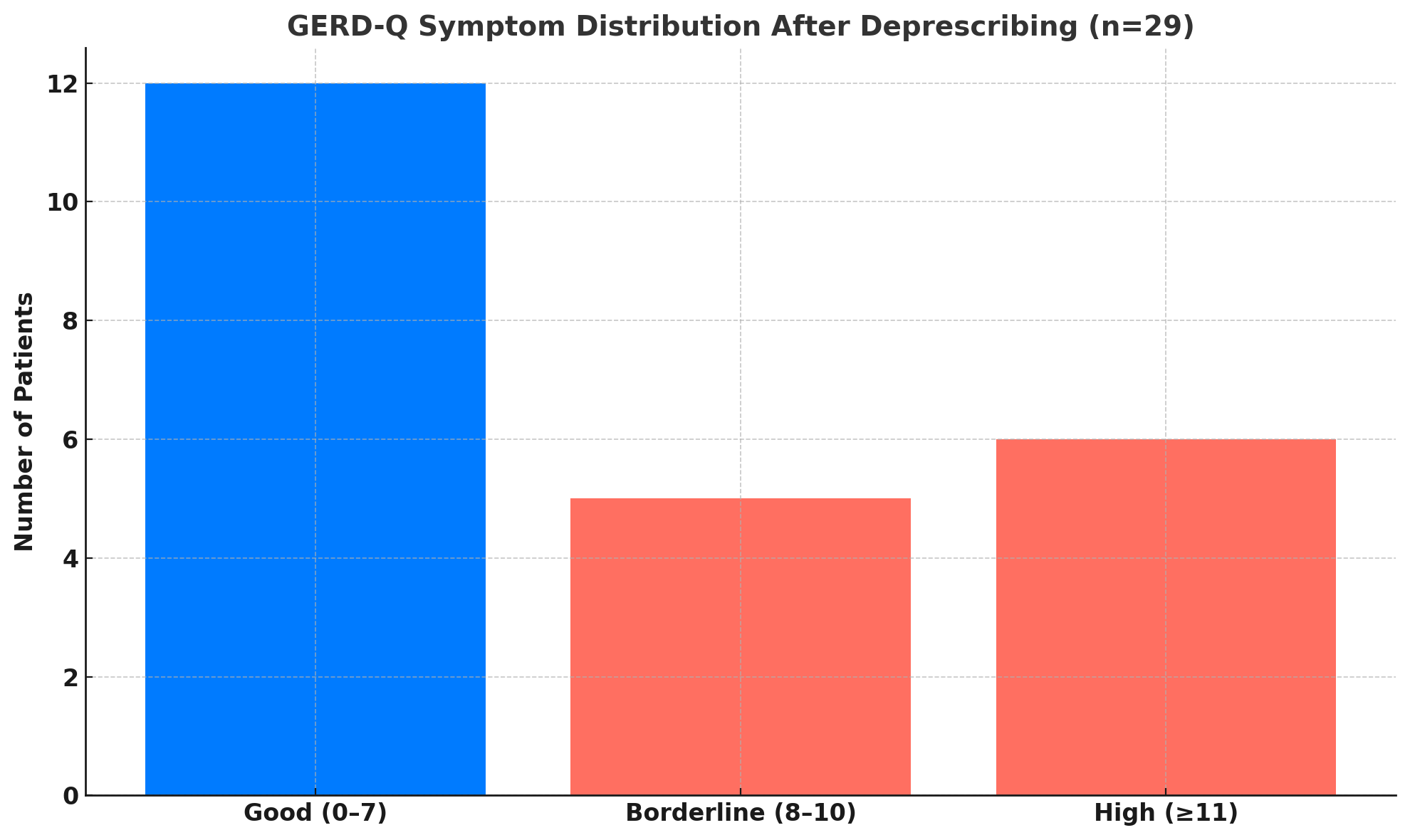
Figure: Pie graph showing de-prescription status of the study population
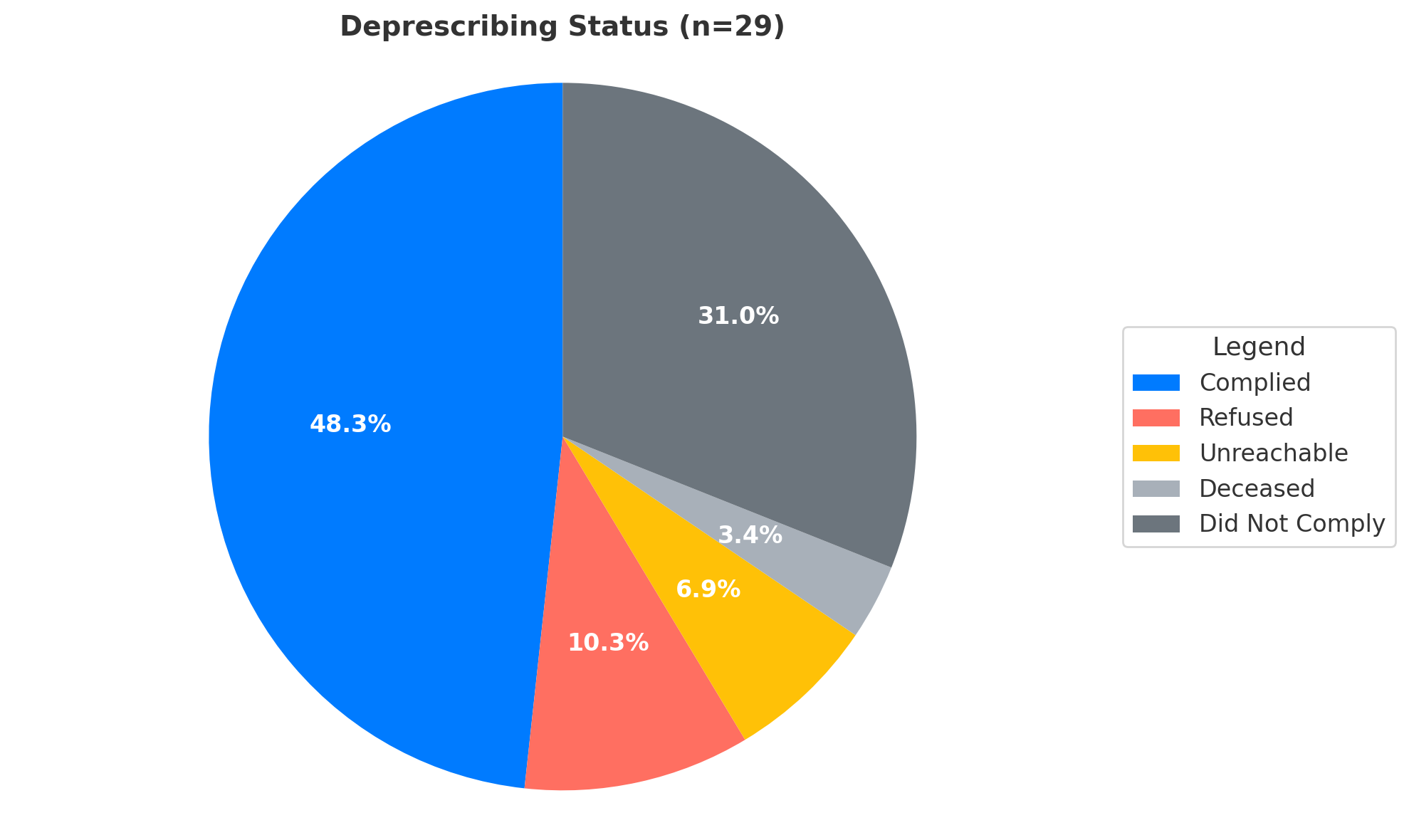
Figure: GERD-Q symptom distribution after deprescription
Disclosures:
Warsha Korani indicated no relevant financial relationships.
Sanjana Kamat indicated no relevant financial relationships.
Darpan Sohni indicated no relevant financial relationships.
Raaghvi Kohli indicated no relevant financial relationships.
Laiba Khan indicated no relevant financial relationships.
Euzebio Ribeiro Silva indicated no relevant financial relationships.
Saba Waseem indicated no relevant financial relationships.
Warsha Korani, MD1, Sanjana Kamat, 2, Darpan Sohni, 2, Raaghvi Kohli, MD3, Laiba Khan, 2, Euzebio Ribeiro Silva, 2, Saba Waseem, 2. P0647 - De-Escalating Proton Pump Inhibitor Use in Outpatient Setting – A Quality Improvement Venture, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Conemaugh Memorial Medical Center, Johnstown, PA; 2Conemaugh memorial medical center, Johnstown, PA; 3Conemaugh Memorial Medical Centre, Johnstown, PA
Introduction:
Proton pump inhibitors (PPIs) are among the most commonly used medications for acid-related gastrointestinal diseases, including GERD, PUD, Barrett’s esophagus, and Zollinger-Ellison syndrome. However, chronic use is associated with increased risks of fractures, chronic kidney disease, Clostridioides difficile infection, hypomagnesemia, and vitamin B12 deficiency. Despite guideline recommendations from the American Gastroenterological Association (AGA) and American College of Gastroenterology (ACG) for regular review and deprescription, many patients continue PPI therapy indefinitely without reassessment or appropriate endoscopic evaluation.
Methods: Patients aged ≥18 years on long-term PPI therapy were identified during routine clinic visits. Exclusion criteria included Barrett’s esophagus, peptic ulcer disease, upper gastrointestinal bleeding, or chronic high-dose NSAID use. Standardized educational materials were provided to patients outlining the risks of chronic PPI use. Deprescribing strategies included dose down-titration, transition to H2 receptor antagonists, or as-needed therapy. Outcomes assessed included symptom control using the GERD-Q questionnaire, rescue medication use, compliance with deprescribing, and prior history of endoscopic evaluation.
Results: A total of 29 patients were reviewed. The median PPI duration was 4 years. Despite long-term therapy, only 13 patients (44.8%) had previously undergone esophagogastroduodenoscopy (EGD), falling short of ACG guidelines recommending endoscopy after 8 weeks of refractory symptoms. Fourteen patients (48.3%) successfully complied with deprescribing. Three patients (10.3%) refused, and two (6.9%) were unreachable for follow-up. At follow-up, 50% had good symptom control (GERD-Q ≤7), 16.7% had borderline symptoms (8–10), and 25% had significant symptoms (≥11). Rescue medication use was reported in 41.7% of cases, with nausea being the most common post-deprescribing symptom. No serious adverse events occurred.
Discussion: This preliminary analysis highlights that nearly half of patients could be successfully deprescribed, with no major adverse events. The majority had been on PPIs for years without appropriate endoscopic evaluation. As additional patients are followed in the coming weeks, this project underscores the urgent need to integrate deprescribing protocols, enhance physician awareness, and engage patients through education in order to optimize the safe use of PPIs in outpatient care

Figure: Pie graph showing de-prescription status of the study population

Figure: GERD-Q symptom distribution after deprescription
Disclosures:
Warsha Korani indicated no relevant financial relationships.
Sanjana Kamat indicated no relevant financial relationships.
Darpan Sohni indicated no relevant financial relationships.
Raaghvi Kohli indicated no relevant financial relationships.
Laiba Khan indicated no relevant financial relationships.
Euzebio Ribeiro Silva indicated no relevant financial relationships.
Saba Waseem indicated no relevant financial relationships.
Warsha Korani, MD1, Sanjana Kamat, 2, Darpan Sohni, 2, Raaghvi Kohli, MD3, Laiba Khan, 2, Euzebio Ribeiro Silva, 2, Saba Waseem, 2. P0647 - De-Escalating Proton Pump Inhibitor Use in Outpatient Setting – A Quality Improvement Venture, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
