Sunday Poster Session
Category: Esophagus
P0644 - Diagnostic Accuracy of Non-Endoscopic Cell Collection Devices for Barrett’s Esophagus: A Systematic Review and Meta-Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Rohit Goyal, MD
Louisiana State University
Shreveport, LA
Presenting Author(s)
Rohit Goyal, MD1, Karan Sachdeva, MD2, Viraj Panchal, MBBS3, Prasad Iyer, MD, MS4
1Louisiana State University, Shreveport, LA; 2LSU Health, Shreveport, LA; 3Louisiana State University Health, Shreveport, LA; 4Mayo Clinic, Phoenix, AZ
Introduction: Barrett's esophagus (BE) is the only known precursor for esophageal adenocarcinoma (EAC). Early detection and treatment of BE are crucial in preventing progression to EAC. Endoscopic screening is suggested, but uptake rates are low. Non-endoscopic screening tests combining swallowable esophageal cell collection devices with biomarkers such as TFF3 and methylated DNA markers (MDMs) have been guideline-endorsed as alternatives to endoscopy. We aimed to evaluate the diagnostic accuracy of these non-endoscopic screening tests in a systematic review and meta-analysis.
Methods: We conducted a comprehensive literature search using PubMed, Embase, and Google Scholar to identify all the published studies analyzing the diagnostic accuracy of different non-endoscopic molecular BE screening tests, using the endoscopy as the comparator. We excluded studies using samples from prior trials or assessing post-ablation surveillance. Two reviewers independently screened titles, abstracts, and full texts. A random-effects meta-analysis was conducted to pool estimates of sensitivity and specificity, and heterogeneity was assessed using the I² statistic.
Results: Out of 148 initial citations, 16 studies met inclusion criteria (Figure 1). These included six studies on Cytosponge (four testing for TFF3, one combining TFF3 with p53, and one evaluating seven biomarkers), five studies on EsoCheck (testing for methylation on the VIM and CCNA1 genes), and five studies on EsophaCap (four testing for Methylated DNA Markers and one testing for Cytology and MUC2 IHC). The pooled sensitivity for the diagnosis of BE was 82.8% (95% confidence interval (CI) = 78.3% - 86.6%), and the pooled specificity was 86.7% (95% CI = 78.0% - 92.2%). The forest plots for the pooled sensitivity and specificity are shown in Figure 2.
Discussion: Non-endoscopic cell collection devices demonstrate high diagnostic accuracy for Barrett's esophagus and represent promising, minimally invasive alternatives to endoscopy. These tools could facilitate earlier detection and broaden access to screening in primary care and underserved settings.
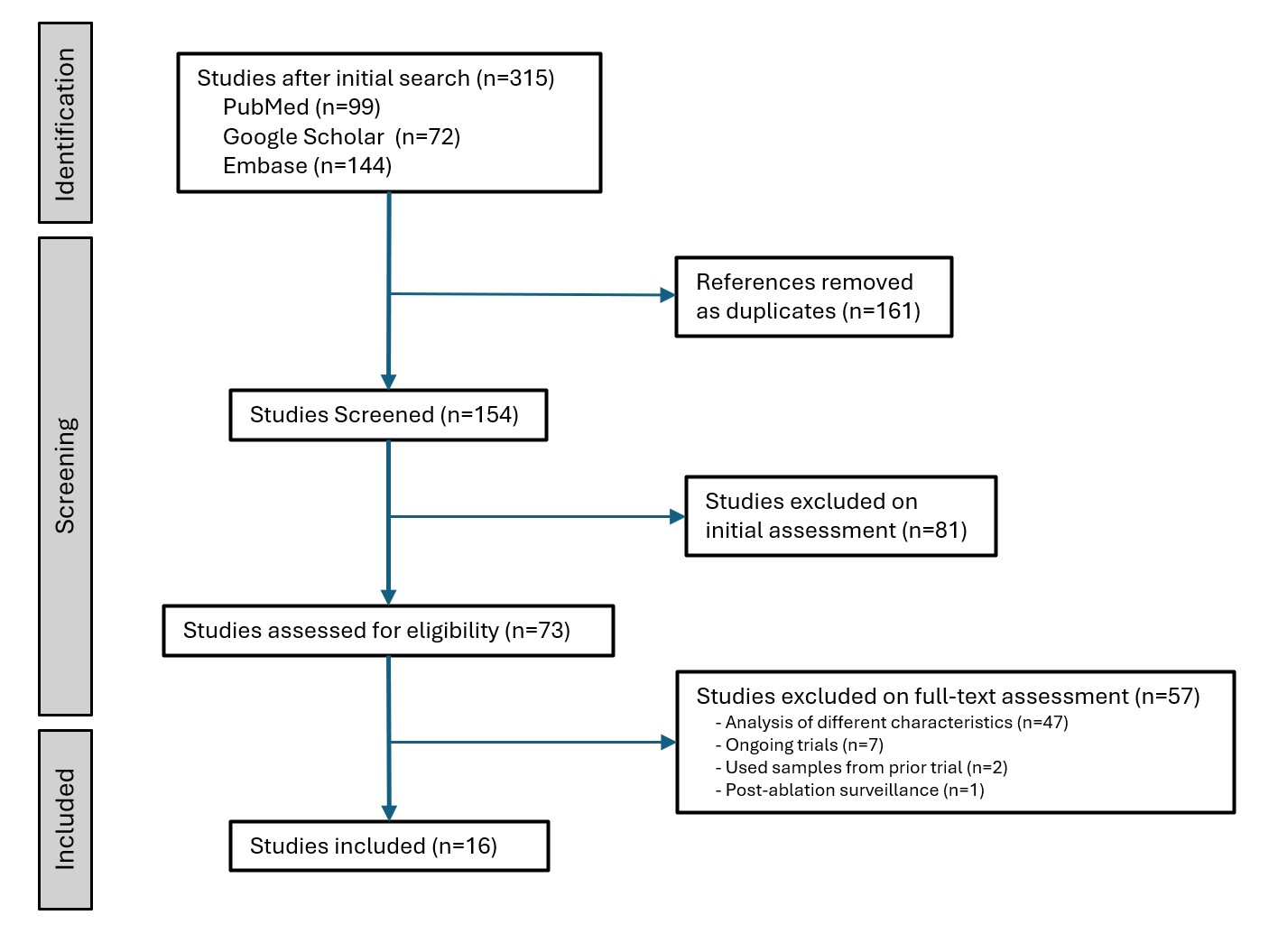
Figure: Figure 1: PRISMA flow diagram showing the inclusion of studies
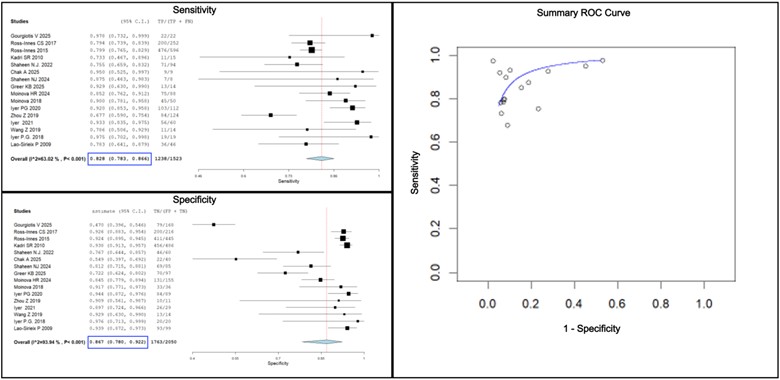
Figure: Figure 2: Forest plots for sensitivity and specificity for the diagnosis of BE using non-endoscopic cell collection devices, using endoscopy with biopsy as the reference standard
Disclosures:
Rohit Goyal indicated no relevant financial relationships.
Karan Sachdeva indicated no relevant financial relationships.
Viraj Panchal indicated no relevant financial relationships.
Prasad Iyer: CDx medical – Consultant, Grant/Research Support. Exact – Grant/Research Support. Exact Sciences – Consultant, Grant/Research Support. Medtronic – Consultant. Pentax Medical – Consultant, Grant/Research Support.
Rohit Goyal, MD1, Karan Sachdeva, MD2, Viraj Panchal, MBBS3, Prasad Iyer, MD, MS4. P0644 - Diagnostic Accuracy of Non-Endoscopic Cell Collection Devices for Barrett’s Esophagus: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Louisiana State University, Shreveport, LA; 2LSU Health, Shreveport, LA; 3Louisiana State University Health, Shreveport, LA; 4Mayo Clinic, Phoenix, AZ
Introduction: Barrett's esophagus (BE) is the only known precursor for esophageal adenocarcinoma (EAC). Early detection and treatment of BE are crucial in preventing progression to EAC. Endoscopic screening is suggested, but uptake rates are low. Non-endoscopic screening tests combining swallowable esophageal cell collection devices with biomarkers such as TFF3 and methylated DNA markers (MDMs) have been guideline-endorsed as alternatives to endoscopy. We aimed to evaluate the diagnostic accuracy of these non-endoscopic screening tests in a systematic review and meta-analysis.
Methods: We conducted a comprehensive literature search using PubMed, Embase, and Google Scholar to identify all the published studies analyzing the diagnostic accuracy of different non-endoscopic molecular BE screening tests, using the endoscopy as the comparator. We excluded studies using samples from prior trials or assessing post-ablation surveillance. Two reviewers independently screened titles, abstracts, and full texts. A random-effects meta-analysis was conducted to pool estimates of sensitivity and specificity, and heterogeneity was assessed using the I² statistic.
Results: Out of 148 initial citations, 16 studies met inclusion criteria (Figure 1). These included six studies on Cytosponge (four testing for TFF3, one combining TFF3 with p53, and one evaluating seven biomarkers), five studies on EsoCheck (testing for methylation on the VIM and CCNA1 genes), and five studies on EsophaCap (four testing for Methylated DNA Markers and one testing for Cytology and MUC2 IHC). The pooled sensitivity for the diagnosis of BE was 82.8% (95% confidence interval (CI) = 78.3% - 86.6%), and the pooled specificity was 86.7% (95% CI = 78.0% - 92.2%). The forest plots for the pooled sensitivity and specificity are shown in Figure 2.
Discussion: Non-endoscopic cell collection devices demonstrate high diagnostic accuracy for Barrett's esophagus and represent promising, minimally invasive alternatives to endoscopy. These tools could facilitate earlier detection and broaden access to screening in primary care and underserved settings.

Figure: Figure 1: PRISMA flow diagram showing the inclusion of studies

Figure: Figure 2: Forest plots for sensitivity and specificity for the diagnosis of BE using non-endoscopic cell collection devices, using endoscopy with biopsy as the reference standard
Disclosures:
Rohit Goyal indicated no relevant financial relationships.
Karan Sachdeva indicated no relevant financial relationships.
Viraj Panchal indicated no relevant financial relationships.
Prasad Iyer: CDx medical – Consultant, Grant/Research Support. Exact – Grant/Research Support. Exact Sciences – Consultant, Grant/Research Support. Medtronic – Consultant. Pentax Medical – Consultant, Grant/Research Support.
Rohit Goyal, MD1, Karan Sachdeva, MD2, Viraj Panchal, MBBS3, Prasad Iyer, MD, MS4. P0644 - Diagnostic Accuracy of Non-Endoscopic Cell Collection Devices for Barrett’s Esophagus: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
