Sunday Poster Session
Category: Endoscopy Video Forum
P0588 - Calcium Stimulation-Guided Endoscopic Ultrasound Radiofrequency Ablation for Symptomatic Metastatic Insulinoma in a Patient With MEN1
- KT
Kathryn Thompson, MD
Emory University School of Medicine
Atlanta, GA
Presenting Author(s)
Emory University School of Medicine, Atlanta, GA
Introduction:
Insulinomas are rare neuroendocrine tumors (NET) that cause recurrent fasting hypoglycemia and neuroglycopenic symptoms. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) is a novel, minimally invasive treatment for insulinomas that causes cell death with targeted thermal energy and has been used as an alternative to surgical resection for management of hypoglycemia.
Case Description/
Methods:
A 41-year-old female with multiple endocrine neoplasia type 1 presented with a 10-year history of recurrent fasting hypoglycemia. Fasting labs revealed blood glucose 54 mg/dL, insulin 3.8 mIU/L, proinsulin 9.8 pmol/L, and C-peptide 1.8 ng/mL. CT abdomen/pelvis demonstrated a large 2.4 x 2.5 x 2.9 cm well-circumscribed pancreatic head mass and three pancreatic body and tail lesions (Figures 1A-D). Dotatate PET/CT revealed a somatostatin avid pancreatic head mass, pancreatic tail nodules, and multiple hepatic, retroperitoneal, and peripancreatic lesions consistent with metastatic insulinoma (Figures 1E-F). EUS revealed a 31 mm x 27 mm pancreatic head mass, 12 mm pancreatic tail lesion, and sub-centimeter left hepatic lesion (Figures 1G-I). Fine needle aspiration of the pancreatic head mass confirmed well-differentiated NET (Figures 2A-B). A selective arterial calcium stimulation test (SACST) identified the source of pathologic insulin production in the pancreatic body/tail despite the dominant lesion in the head (Figure 2C). She underwent EUS-RFA with five ablations of an 11 mm lesion in the pancreatic body for 11-18 seconds, five ablations of an 11 mm lesion in the pancreatic tail for 15-22 seconds, and two ablations of an 8 mm lesion in the pancreatic tail for 16-17 seconds using a 10 mm trocar. One day after EUS-RFA, fasting labs revealed blood glucose 117 mg/dL, insulin 4.8 mIU/L, C-peptide 2.6 ng/mL, and she reported no episodes of hypoglycemia at follow-up. Two months after EUS-RFA, MRI abdomen/pelvis demonstrated small pancreatic body and tail lesions without enhancement at sites of ablation and unchanged pancreatic head mass and hepatic, retroperitoneal, and peripancreatic lesions (Figures 2D-F).
Discussion:
In a patient with metastatic insulinoma, the SACST was successful in localizing the source of pathologic insulin production to guide treatment with EUS-RFA. Despite limited reports of EUS-RFA for insulinomas and none in the setting of metastatic disease, this modality appears to be a promising potential approach, in this case guided by the SACST to target sites of disease.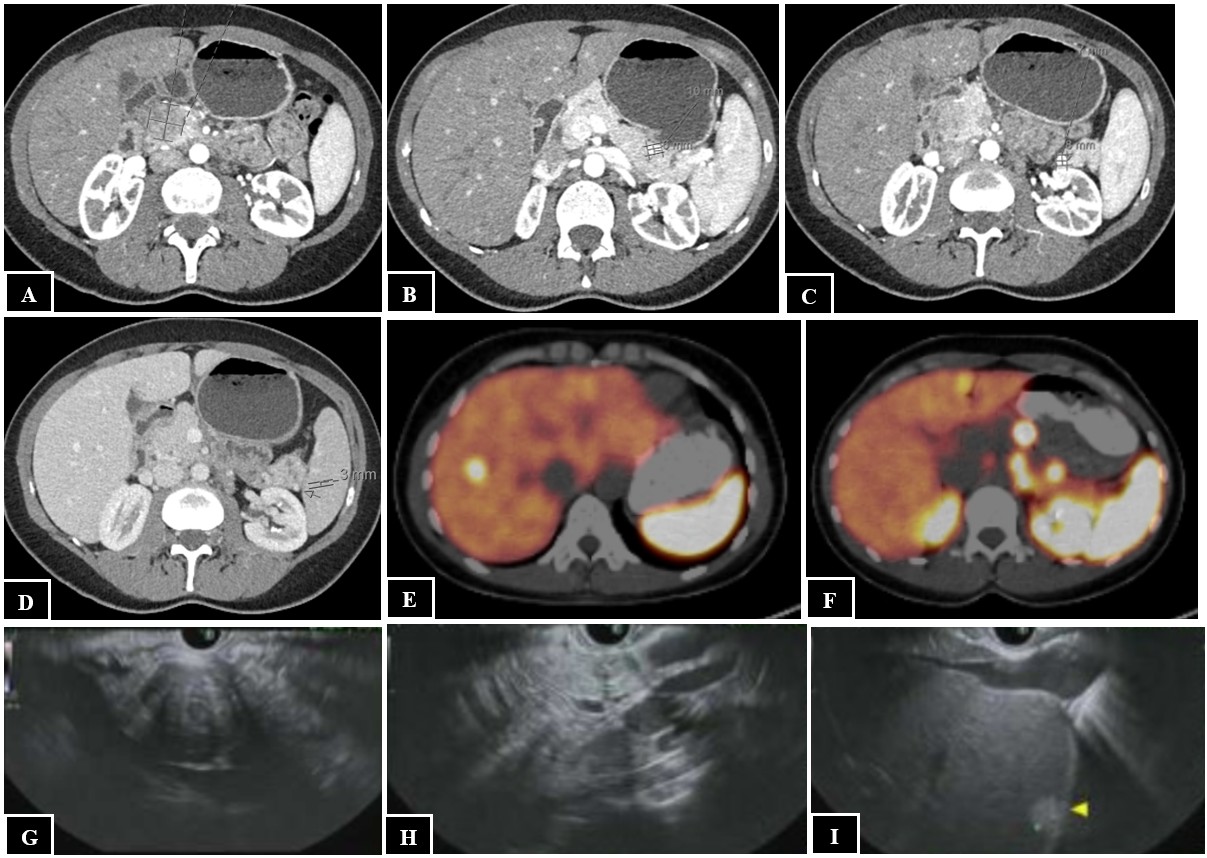
Figure: Figure 1. CT abdomen/pelvis showing a 2.4 x 2.5 by 2.9 cm well-circumscribed pancreatic head mass (A) and three pancreatic body and tail lesions (B-D). Dotatate PET/CT showing a somatostatin avid pancreatic head mass (E), pancreatic tail nodules (E), and hepatic lesion (F). EUS showing a pancreatic head mass (G), pancreatic tail lesion (H), and hepatic lesion (I).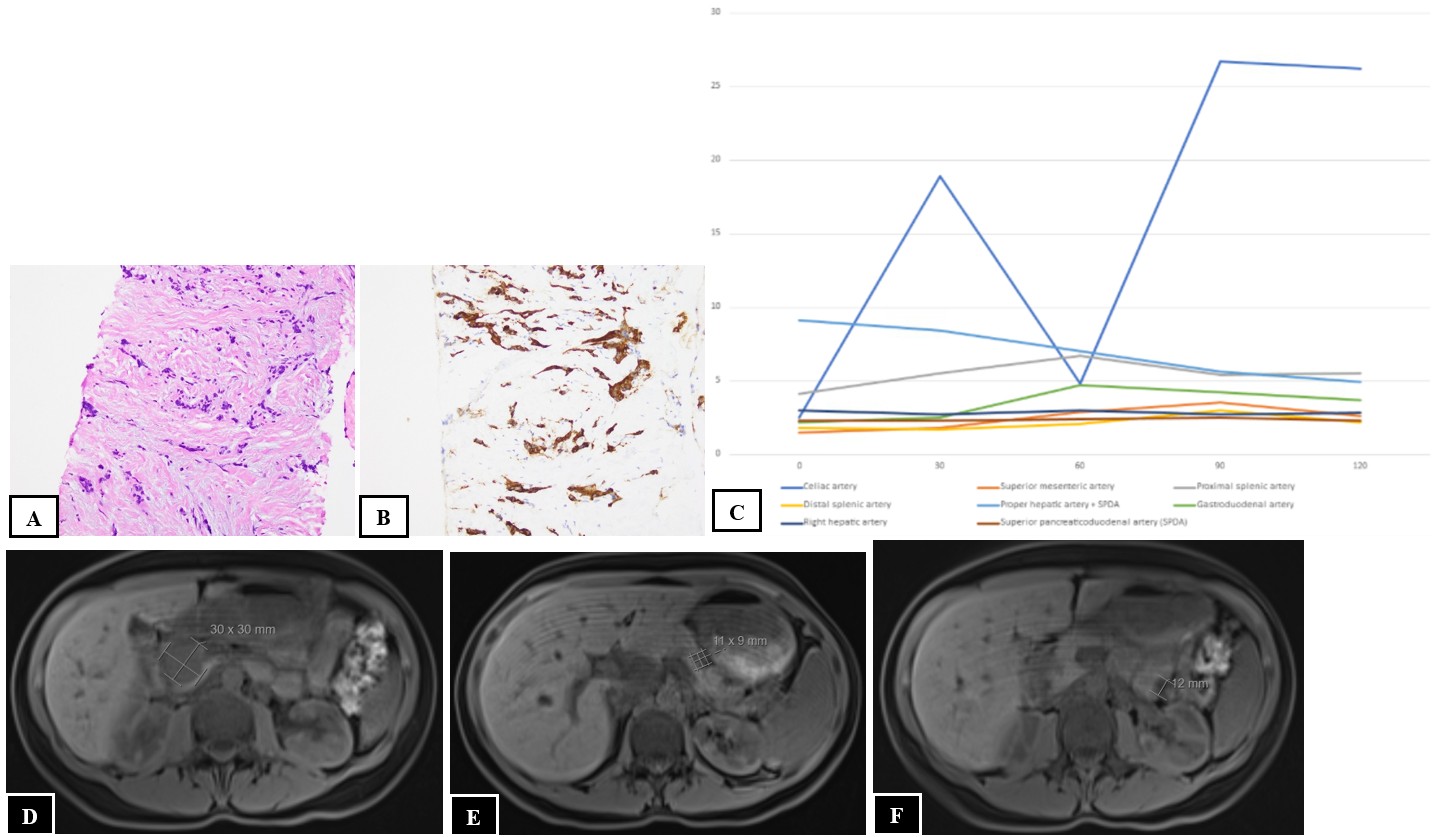
Figure: Figure 2. Histopathology of fine needle aspiration of the pancreatic head mass confirming well-differentiated NET, including hematoxylin and eosin stain at 20x magnification (A) and synaptophysin immunohistochemical stain at 20x magnification (B). SACST results with time (seconds) on the x-axis and insulin level (mIU/L) on the y-axis, which identified the source of pathologic insulin production in the pancreatic body/tail based on insulin concentration increase of greater than 2.7-fold over a 30-second period after calcium stimulation in the celiac artery. The sudden drop at the 60-second mark was felt to be the result of a sampling error due to respiratory motion. The 30-, 90-, and 120-second measurements are consistent with NET stimulation in this distribution (C). MRI abdomen pelvis two months post-EUS-RFA showing an unchanged pancreatic head mass (D) and small pancreatic body (E) and tail (F) lesions without enhancement at sites of ablation.
Disclosures:
Kathryn Thompson indicated no relevant financial relationships.
Zachary Bercu indicated no relevant financial relationships.
Daniel Halperin: Abbvie – Advisor or Review Panel Member, Consultant. Advanced Accelerator Applications – Grant/Research Support. Amryt Pharma – Advisor or Review Panel Member, Consultant. Boehringer Ingelheim – Advisor or Review Panel Member, Consultant. Camurus – Advisor or Review Panel Member, Consultant, Grant/Research Support. Chimeric Therapeutics – Advisor or Review Panel Member, Consultant, Grant/Research Support. Crinetics Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support. Exelixis – Advisor or Review Panel Member, Consultant. Genentech/Roche – Grant/Research Support. Harbour BioMed – Advisor or Review Panel Member, Consultant. Harpoon therapeutics – Advisor or Review Panel Member, Consultant. Ipsen – Advisor or Review Panel Member, Consultant. ITM – Advisor or Review Panel Member, Consultant, Grant/Research Support. Lantheus Medical Imaging – Advisor or Review Panel Member, Consultant. Novartis – Advisor or Review Panel Member, Consultant. Progenics – Advisor or Review Panel Member, Consultant. Radiomedix – Advisor or Review Panel Member, Consultant. RayzeBio – Grant/Research Support. Sanofi Aventis GmbH – Advisor or Review Panel Member, Consultant. Tarveda Therapeutics – Grant/Research Support. Tersera – Advisor or Review Panel Member, Consultant. Thermo Fisher Scientific – Grant/Research Support.
Priya Dayamani John indicated no relevant financial relationships.
Saurabh Chawla indicated no relevant financial relationships.
Field Willingham indicated no relevant financial relationships.
Kathryn Thompson, MD, Zachary Bercu, MD, Daniel Halperin, MD, Priya Dayamani John, MD, Saurabh Chawla, MD, FACG, Field Willingham, MD. P0588 - Calcium Stimulation-Guided Endoscopic Ultrasound Radiofrequency Ablation for Symptomatic Metastatic Insulinoma in a Patient With MEN1, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

