Sunday Poster Session
Category: Diet, Nutrition, and Obesity
P0557 - Risk of Vision-Threatening Eye Complications Among GLP-1 Receptor Agonist Users: A Retrospective Cohort Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- SL
Samira Lemos, MD
Yaoundé General Hospital
Yaoundé, Sud, Cameroon
Presenting Author(s)
Sarpong Boateng, MD, MPH1, Samira Lemos, MD2, Prince A. Ameyaw, MD3, Guy Loic Nguefang Tchoukeu, MD4, Ikechukwu Elvis Eze, MBBS5, Chimezirim Ezeano, MD, MPH6, Thiruvengadam Muniraj, MD7, Basile Njei, MD, PhD, MPH8, Shambavi Annush, BS9
1Yale New Haven Health, Bridgeport, CT; 2Yaoundé General Hospital, Yaoundé, Sud, Cameroon; 3Yale New Haven Health, Bridgeport Hospital, Bridgeport, CT; 4Texas Tech University Health Sciences Center, Odessa, TX; 5Yale New Haven Hospital / Yale University, New Haven, CT; 6Aurora Healthcare, Aurora, WI; 7Yale University School of Medicine, New Haven, CT; 8VA Connecticut Healthcare System and Yale University, West Haven, CT; 9Stanley Medical College, Chennai, Tamil Nadu, India
Introduction: GLP-1 receptor agonists (GLP-1 RAs) are widely prescribed for type 2 DM (T2DM) and obesity, with well-established cardiometabolic benefits. However, emerging reports have raised concerns about ocular complications, particularly ischemic optic neuropathy, optic papillitis, and maculopathy. While the mechanisms remain uncertain, recent evidence suggests that rapid glycemic reduction and immune modulation may contribute to retinal injury. This study assessed the relationship between GLP-1 RA use and vision-threatening eye complications in a large, diverse U.S. cohort.
Methods: We conducted a retrospective cohort study using electronic health record data from the NIH All of Us Research Program (2016–2022). Adults with obesity or T2DM were included; individuals with prior eye malignancy or advanced retinal disease were excluded. Eye complications were defined as a composite of ischemic optic neuropathy, optic papillitis, or maculopathy. A 1:1 propensity score matching was used to balance covariates between GLP-1 RA users and non-users based on demographics. Multivariable logistic regression estimated adjusted odds ratios (aORs), controlling for BMI, comorbidities, HbA1c and medications.
Results: Among 34,380 matched participants (n=17,190 per group; mean age 59.3 yrs; 66.6% female), overall eye complications were more common in GLP-1 RA users (4.8% vs. 1.3%; p < 0.001). In adjusted analysis, GLP-1 RA use was associated with increased odds of eye complications (aOR: 1.58, 95% CI: 1.32–1.89, p < 0.001). The association was strongest for maculopathy (aOR: 1.64, 95% CI: 1.36–1.98, p < 0.001), while there was no significant association for ischemic optic neuropathy or optic papillitis. Independent predictors included T2DM (aOR: 5.50, 95% CI: 4.02–7.53, p < 0.001), hypertension (aOR: 1.70, 95% CI: 1.48–1.95, p < 0.001) and autoimmune disease (aOR: 1.36, 95% CI: 1.14–1.62, p = 0.001). A positive association was also noted between HbA1c variability and eye complications.
Discussion: GLP-1 RA use was independently associated with increased odds of maculopathy. The lack of association with ischemic optic neuropathy may reflect the anti-inflammatory properties of GLP-1 RA agents on its arteritic subtype. Our findings support the hypothesis that rapid glycemic shifts may underlie GLP-1–associated retinal injury, in line with prior pharmacovigilance data. Proactive ocular surveillance may be warranted in high-risk GLP-1 RA users, especially those with T2DM, autoimmune conditions, or HbA1c variability.
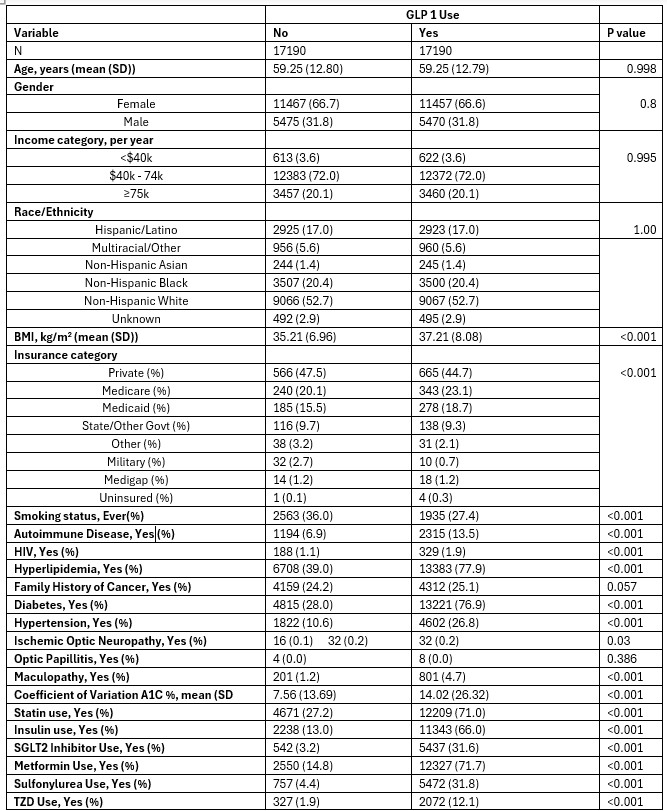
Figure: Table 1: Characteristics of Participants after Propensity Score Matching on Demographics
GLP-1 RA: Glucagon-like peptide-1 receptor agonist; BMI: Body Mass Index; MASLD: Metabolic dysfunction-associated steatotic liver disease; CKD: Chronic kidney disease; SGLT2: Sodium-glucose cotransporter 2; TZD: Thiazolidinedione.
Values are expressed as mean ± standard deviation for continuous variables and percentages for categorical variables.
P-values are derived from t-tests (continuous) or chi-square tests (categorical).
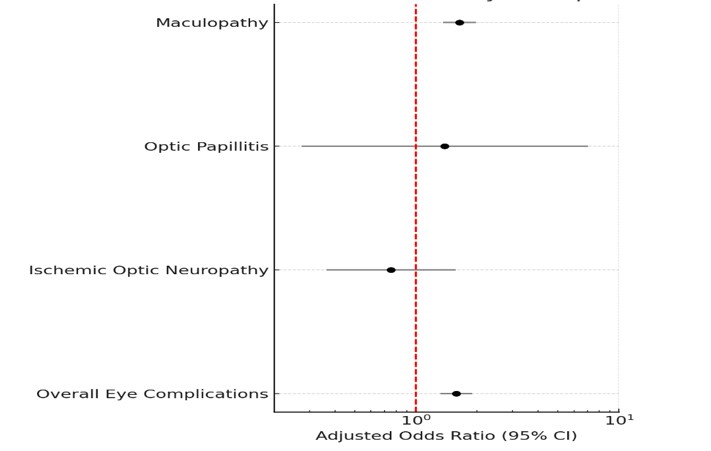
Figure: Figure 1 - GLP-1 Use and Risk of Eye Complications
• OR: Odds Ratio; CI: Confidence Interval.
• PSM: Propensity Score Matching (1:1 ratio).
• The adjusted model includes the following covariates:
Demographic factors: age, sex, race/ethnicity, income.
Clinical characteristics: BMI, A1C variation, MASLD, Diabetes, Hypertension, autoimmune disease, HIV status, chronic kidney disease.
Medication use: insulin, TZDs, statins, SGLT2 inhibitors, metformin, sulfonylureas.
Smoking status: current, former, never, unknown.
Family history: any cancer.
Disclosures:
Sarpong Boateng indicated no relevant financial relationships.
Samira Lemos indicated no relevant financial relationships.
Prince Ameyaw indicated no relevant financial relationships.
Guy Loic Nguefang Tchoukeu indicated no relevant financial relationships.
Ikechukwu Elvis Eze indicated no relevant financial relationships.
Chimezirim Ezeano indicated no relevant financial relationships.
Thiruvengadam Muniraj indicated no relevant financial relationships.
Basile Njei indicated no relevant financial relationships.
Shambavi Annush indicated no relevant financial relationships.
Sarpong Boateng, MD, MPH1, Samira Lemos, MD2, Prince A. Ameyaw, MD3, Guy Loic Nguefang Tchoukeu, MD4, Ikechukwu Elvis Eze, MBBS5, Chimezirim Ezeano, MD, MPH6, Thiruvengadam Muniraj, MD7, Basile Njei, MD, PhD, MPH8, Shambavi Annush, BS9. P0557 - Risk of Vision-Threatening Eye Complications Among GLP-1 Receptor Agonist Users: A Retrospective Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Yale New Haven Health, Bridgeport, CT; 2Yaoundé General Hospital, Yaoundé, Sud, Cameroon; 3Yale New Haven Health, Bridgeport Hospital, Bridgeport, CT; 4Texas Tech University Health Sciences Center, Odessa, TX; 5Yale New Haven Hospital / Yale University, New Haven, CT; 6Aurora Healthcare, Aurora, WI; 7Yale University School of Medicine, New Haven, CT; 8VA Connecticut Healthcare System and Yale University, West Haven, CT; 9Stanley Medical College, Chennai, Tamil Nadu, India
Introduction: GLP-1 receptor agonists (GLP-1 RAs) are widely prescribed for type 2 DM (T2DM) and obesity, with well-established cardiometabolic benefits. However, emerging reports have raised concerns about ocular complications, particularly ischemic optic neuropathy, optic papillitis, and maculopathy. While the mechanisms remain uncertain, recent evidence suggests that rapid glycemic reduction and immune modulation may contribute to retinal injury. This study assessed the relationship between GLP-1 RA use and vision-threatening eye complications in a large, diverse U.S. cohort.
Methods: We conducted a retrospective cohort study using electronic health record data from the NIH All of Us Research Program (2016–2022). Adults with obesity or T2DM were included; individuals with prior eye malignancy or advanced retinal disease were excluded. Eye complications were defined as a composite of ischemic optic neuropathy, optic papillitis, or maculopathy. A 1:1 propensity score matching was used to balance covariates between GLP-1 RA users and non-users based on demographics. Multivariable logistic regression estimated adjusted odds ratios (aORs), controlling for BMI, comorbidities, HbA1c and medications.
Results: Among 34,380 matched participants (n=17,190 per group; mean age 59.3 yrs; 66.6% female), overall eye complications were more common in GLP-1 RA users (4.8% vs. 1.3%; p < 0.001). In adjusted analysis, GLP-1 RA use was associated with increased odds of eye complications (aOR: 1.58, 95% CI: 1.32–1.89, p < 0.001). The association was strongest for maculopathy (aOR: 1.64, 95% CI: 1.36–1.98, p < 0.001), while there was no significant association for ischemic optic neuropathy or optic papillitis. Independent predictors included T2DM (aOR: 5.50, 95% CI: 4.02–7.53, p < 0.001), hypertension (aOR: 1.70, 95% CI: 1.48–1.95, p < 0.001) and autoimmune disease (aOR: 1.36, 95% CI: 1.14–1.62, p = 0.001). A positive association was also noted between HbA1c variability and eye complications.
Discussion: GLP-1 RA use was independently associated with increased odds of maculopathy. The lack of association with ischemic optic neuropathy may reflect the anti-inflammatory properties of GLP-1 RA agents on its arteritic subtype. Our findings support the hypothesis that rapid glycemic shifts may underlie GLP-1–associated retinal injury, in line with prior pharmacovigilance data. Proactive ocular surveillance may be warranted in high-risk GLP-1 RA users, especially those with T2DM, autoimmune conditions, or HbA1c variability.

Figure: Table 1: Characteristics of Participants after Propensity Score Matching on Demographics
GLP-1 RA: Glucagon-like peptide-1 receptor agonist; BMI: Body Mass Index; MASLD: Metabolic dysfunction-associated steatotic liver disease; CKD: Chronic kidney disease; SGLT2: Sodium-glucose cotransporter 2; TZD: Thiazolidinedione.
Values are expressed as mean ± standard deviation for continuous variables and percentages for categorical variables.
P-values are derived from t-tests (continuous) or chi-square tests (categorical).

Figure: Figure 1 - GLP-1 Use and Risk of Eye Complications
• OR: Odds Ratio; CI: Confidence Interval.
• PSM: Propensity Score Matching (1:1 ratio).
• The adjusted model includes the following covariates:
Demographic factors: age, sex, race/ethnicity, income.
Clinical characteristics: BMI, A1C variation, MASLD, Diabetes, Hypertension, autoimmune disease, HIV status, chronic kidney disease.
Medication use: insulin, TZDs, statins, SGLT2 inhibitors, metformin, sulfonylureas.
Smoking status: current, former, never, unknown.
Family history: any cancer.
Disclosures:
Sarpong Boateng indicated no relevant financial relationships.
Samira Lemos indicated no relevant financial relationships.
Prince Ameyaw indicated no relevant financial relationships.
Guy Loic Nguefang Tchoukeu indicated no relevant financial relationships.
Ikechukwu Elvis Eze indicated no relevant financial relationships.
Chimezirim Ezeano indicated no relevant financial relationships.
Thiruvengadam Muniraj indicated no relevant financial relationships.
Basile Njei indicated no relevant financial relationships.
Shambavi Annush indicated no relevant financial relationships.
Sarpong Boateng, MD, MPH1, Samira Lemos, MD2, Prince A. Ameyaw, MD3, Guy Loic Nguefang Tchoukeu, MD4, Ikechukwu Elvis Eze, MBBS5, Chimezirim Ezeano, MD, MPH6, Thiruvengadam Muniraj, MD7, Basile Njei, MD, PhD, MPH8, Shambavi Annush, BS9. P0557 - Risk of Vision-Threatening Eye Complications Among GLP-1 Receptor Agonist Users: A Retrospective Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
