Sunday Poster Session
Category: Colorectal Cancer Prevention
P0512 - Adenoma Detection Rates from a Large Clinical Validation Study of a Blood-Based Colorectal Cancer Screening Test
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Man Yee Wong, MBBS, MPH, MBA (she/her/hers)
Freenome
San Ramon, CA
Presenting Author(s)
Award: ACG Presidential Poster Award
Man Yee Wong, MBBS, MPH, MBA1, Andy Piscitello, 2, Sarah Kinnings, BSc, MSc, PhD3, Kristen Petersen, MS2, Mahan Matin, MD4, Reinier G.S.. Meester, MS, PhD5, Lilian C.. Lee, PhD2, Aasma Shaukat, MD, MPH, FACG6
1Freenome, San Ramon, CA; 2Freenome, Brisbane, CA; 3Freenome Holdings, Inc., San Diego, CA; 4Freenome, Walnut Creek, CA; 5Freenome Inc, South San Francisco, CA; 6NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY
Introduction: Colorectal cancer (CRC) is the second most common cause of cancer deaths in the United States. Colonoscopy can reduce CRC mortality by enabling earlier cancer detection or by removing cancer precursors. Higher adenoma detection rates (ADRs) have been associated with lower CRC mortality. ADR is a leading colonoscopy quality indicator, but ADR data for adults aged 45-49yrs are limited. This study evaluates ADRs by age for a large real-world cohort of average-risk adults against the 2024 colonoscopy quality guidelines by the American College of Gastroenterology (ACG) and the American Society for Gastrointestinal Endoscopy (ASGE), providing robust, generalizable estimates for the CRC screening population.
Methods: PREEMPT CRC was an observational, prospective clinical validation study of a blood-based test for CRC screening among asymptomatic, average-risk adults aged 45-85yrs. The study included 201 diverse sites across the U.S. and abroad, with screening colonoscopies conducted by more than 5,000 endoscopists. Based on a thorough medical review of colonoscopy and pathology reports, ADRs were calculated across 27,010 subjects who completed screening colonoscopy, and compared to other studies and the ADR performance target of 35% overall, with 40% in men and 30% in women. Overall and sex-stratified ADRs were also calculated across 5-year age brackets.
Results: The overall ADR for 45-85yrs was 36.4% (35.8%, 37.0%) (Figure 1). A significant difference (P < 0.0001) was observed between male subjects (43.5%, 42.6%-44.4%) and female subjects (30.8%, 30.0%-31.5%) across all age brackets. ADRs generally increased with age, with the steepest increase from 45-59yrs (Figure 2). The ADR for 45-49yrs (28.1%, 26.5%-29.8%) was consistent with two recent studies (Shaukat 2022, Bilal 2022) but below ADR performance targets, whereas the ADRs for 50-54yrs (34.4%, 33.4%-35.5%) and 50-75yrs (37.3%, 36.7%-37.9%) were at or above ADR performance targets. These observations held true when stratifying ADR by sex.
Discussion: ADRs for 45-85yrs in PREEMPT CRC were above overall and sex-specific ADR performance targets. Despite lower ADRs for 45-49yrs, adenomas were still detected in more than 1 in 4 screening colonoscopies among this age group, whose screening adherence is generally poor. This key finding underscores the benefit of initiating screening at 45yrs and improving screening adherence in this age group to help reduce CRC incidence and mortality in subsequent decades.
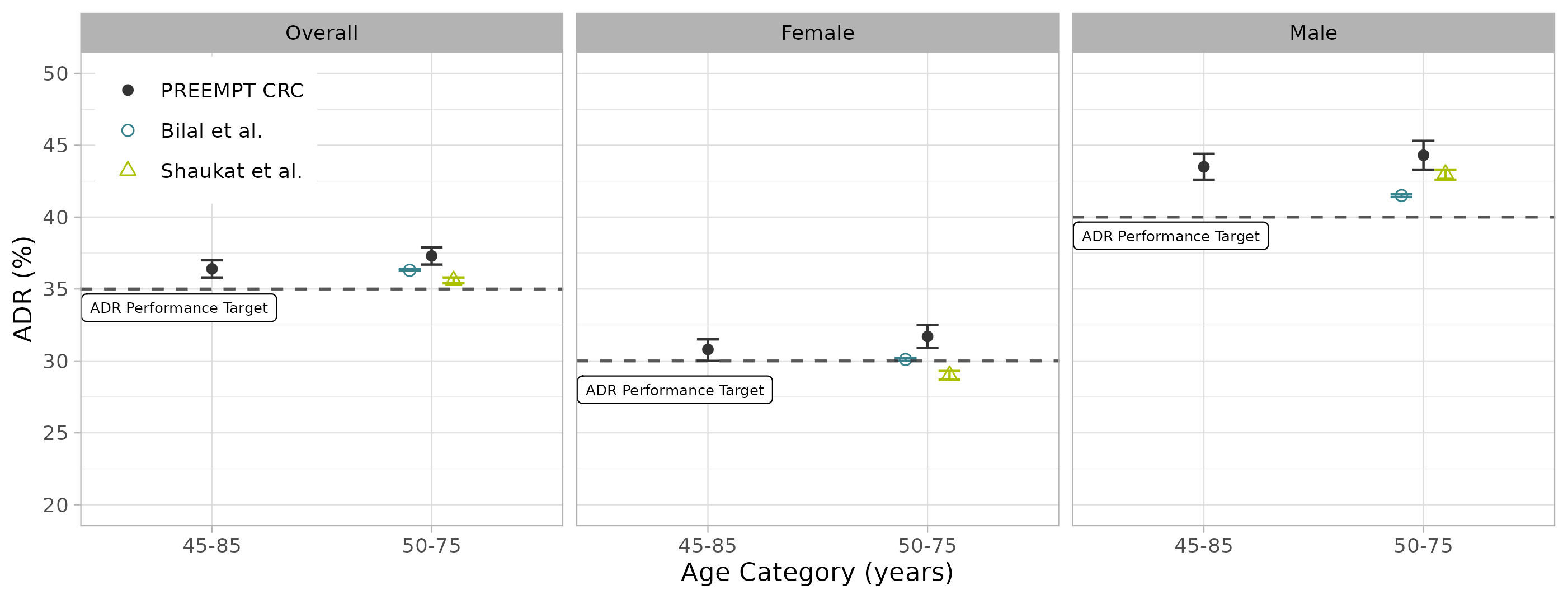
Figure: Figure 1. Comparison of ADRs by age category and sex from PREEMPT CRC versus average ADRs from two other recent studies (Bilal et al., 2022 and Shaukat et al., 2022). 95% confidence intervals for PREEMPT CRC and Bilal et al. were calculated using the Clopper-Pearson method, and for Shaukat et al. they are as reported. The horizontal lines indicate the 35%, 30%, and 40% minimum ADR performance targets for screening colonoscopy for overall, women, and men, respectively.
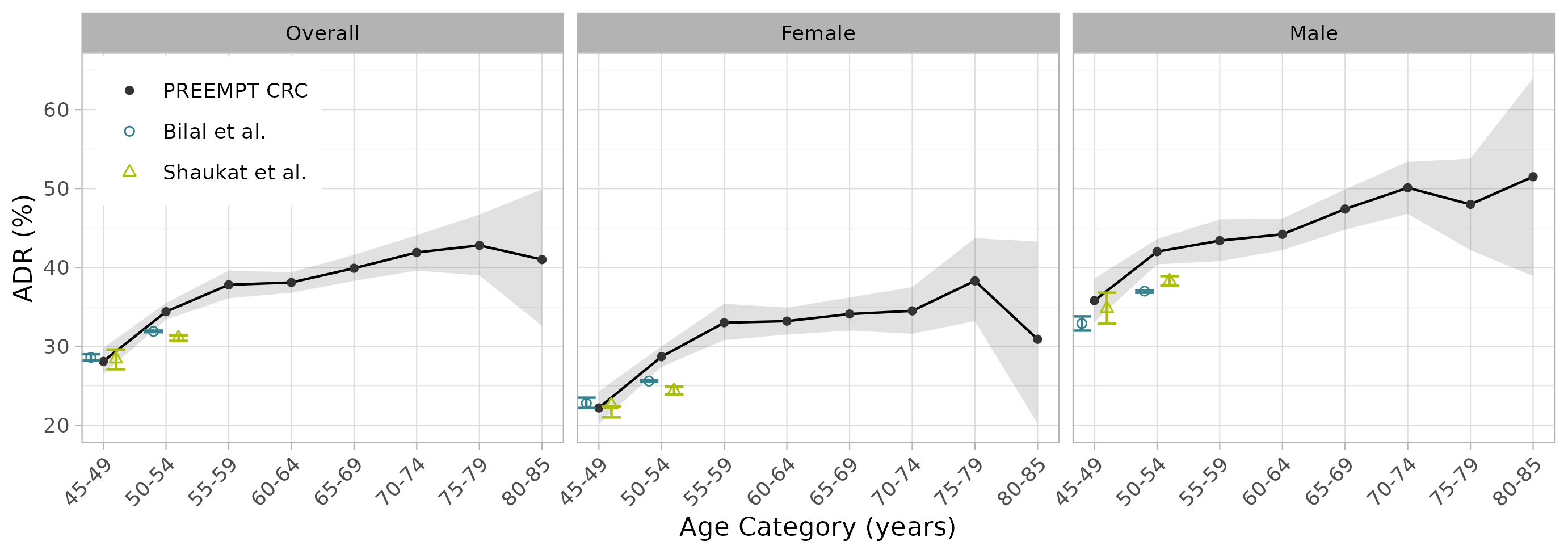
Figure: Figure 2. ADR by 5-year age category for PREEMPT CRC. ADR is shown for each age category overall and stratified by sex. Comparison against average ADRs from two other recent studies (Bilal et al., 2022 and Shaukat et al., 2022) is shown for age categories 45-49yrs and 50-54yrs. 95% confidence intervals for PREEMPT CRC and Bilal et al. were calculated using the Clopper-Pearson method, and for Shaukat et al. they are as reported.
Disclosures:
Man Yee Wong: Freenome Holdings Inc. – Employee, Stock Options, Stock-privately held company. GRAIL, Inc. – Stock-publicly held company(excluding mutual/index funds). Illumina, Inc. – Stock-publicly held company(excluding mutual/index funds). Thermo Fisher Scientific Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Andy Piscitello: Bluestar Genomics – Consultant. Caris Life Sciences – Consultant. Elephas – Consultant. Innovenn – Consultant. Iterative Scopes – Consultant. Karius – Consultant. Medtronic – Consultant. Nucleix – Consultant. Thermo Fisher Scientific – Consultant. Viome – Consultant.
Sarah Kinnings: Freenome Holdings, Inc. – Employee, Stock Options, Stock-privately held company. Grail, Inc. – Stock-publicly held company(excluding mutual/index funds). Illumina, Inc. – Employee, Intellectual Property/Patents, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Kristen Petersen: Freenome – Employee, Stock Options, Stock-privately held company.
Mahan Matin: Freenome – Consultant.
Reinier Meester: Freenome Holdings Inc. – Employee.
Lilian Lee: Freenome – Employee.
Aasma Shaukat: Freenome inc – Consultant.
Man Yee Wong, MBBS, MPH, MBA1, Andy Piscitello, 2, Sarah Kinnings, BSc, MSc, PhD3, Kristen Petersen, MS2, Mahan Matin, MD4, Reinier G.S.. Meester, MS, PhD5, Lilian C.. Lee, PhD2, Aasma Shaukat, MD, MPH, FACG6. P0512 - Adenoma Detection Rates from a Large Clinical Validation Study of a Blood-Based Colorectal Cancer Screening Test, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Man Yee Wong, MBBS, MPH, MBA1, Andy Piscitello, 2, Sarah Kinnings, BSc, MSc, PhD3, Kristen Petersen, MS2, Mahan Matin, MD4, Reinier G.S.. Meester, MS, PhD5, Lilian C.. Lee, PhD2, Aasma Shaukat, MD, MPH, FACG6
1Freenome, San Ramon, CA; 2Freenome, Brisbane, CA; 3Freenome Holdings, Inc., San Diego, CA; 4Freenome, Walnut Creek, CA; 5Freenome Inc, South San Francisco, CA; 6NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY
Introduction: Colorectal cancer (CRC) is the second most common cause of cancer deaths in the United States. Colonoscopy can reduce CRC mortality by enabling earlier cancer detection or by removing cancer precursors. Higher adenoma detection rates (ADRs) have been associated with lower CRC mortality. ADR is a leading colonoscopy quality indicator, but ADR data for adults aged 45-49yrs are limited. This study evaluates ADRs by age for a large real-world cohort of average-risk adults against the 2024 colonoscopy quality guidelines by the American College of Gastroenterology (ACG) and the American Society for Gastrointestinal Endoscopy (ASGE), providing robust, generalizable estimates for the CRC screening population.
Methods: PREEMPT CRC was an observational, prospective clinical validation study of a blood-based test for CRC screening among asymptomatic, average-risk adults aged 45-85yrs. The study included 201 diverse sites across the U.S. and abroad, with screening colonoscopies conducted by more than 5,000 endoscopists. Based on a thorough medical review of colonoscopy and pathology reports, ADRs were calculated across 27,010 subjects who completed screening colonoscopy, and compared to other studies and the ADR performance target of 35% overall, with 40% in men and 30% in women. Overall and sex-stratified ADRs were also calculated across 5-year age brackets.
Results: The overall ADR for 45-85yrs was 36.4% (35.8%, 37.0%) (Figure 1). A significant difference (P < 0.0001) was observed between male subjects (43.5%, 42.6%-44.4%) and female subjects (30.8%, 30.0%-31.5%) across all age brackets. ADRs generally increased with age, with the steepest increase from 45-59yrs (Figure 2). The ADR for 45-49yrs (28.1%, 26.5%-29.8%) was consistent with two recent studies (Shaukat 2022, Bilal 2022) but below ADR performance targets, whereas the ADRs for 50-54yrs (34.4%, 33.4%-35.5%) and 50-75yrs (37.3%, 36.7%-37.9%) were at or above ADR performance targets. These observations held true when stratifying ADR by sex.
Discussion: ADRs for 45-85yrs in PREEMPT CRC were above overall and sex-specific ADR performance targets. Despite lower ADRs for 45-49yrs, adenomas were still detected in more than 1 in 4 screening colonoscopies among this age group, whose screening adherence is generally poor. This key finding underscores the benefit of initiating screening at 45yrs and improving screening adherence in this age group to help reduce CRC incidence and mortality in subsequent decades.

Figure: Figure 1. Comparison of ADRs by age category and sex from PREEMPT CRC versus average ADRs from two other recent studies (Bilal et al., 2022 and Shaukat et al., 2022). 95% confidence intervals for PREEMPT CRC and Bilal et al. were calculated using the Clopper-Pearson method, and for Shaukat et al. they are as reported. The horizontal lines indicate the 35%, 30%, and 40% minimum ADR performance targets for screening colonoscopy for overall, women, and men, respectively.

Figure: Figure 2. ADR by 5-year age category for PREEMPT CRC. ADR is shown for each age category overall and stratified by sex. Comparison against average ADRs from two other recent studies (Bilal et al., 2022 and Shaukat et al., 2022) is shown for age categories 45-49yrs and 50-54yrs. 95% confidence intervals for PREEMPT CRC and Bilal et al. were calculated using the Clopper-Pearson method, and for Shaukat et al. they are as reported.
Disclosures:
Man Yee Wong: Freenome Holdings Inc. – Employee, Stock Options, Stock-privately held company. GRAIL, Inc. – Stock-publicly held company(excluding mutual/index funds). Illumina, Inc. – Stock-publicly held company(excluding mutual/index funds). Thermo Fisher Scientific Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Andy Piscitello: Bluestar Genomics – Consultant. Caris Life Sciences – Consultant. Elephas – Consultant. Innovenn – Consultant. Iterative Scopes – Consultant. Karius – Consultant. Medtronic – Consultant. Nucleix – Consultant. Thermo Fisher Scientific – Consultant. Viome – Consultant.
Sarah Kinnings: Freenome Holdings, Inc. – Employee, Stock Options, Stock-privately held company. Grail, Inc. – Stock-publicly held company(excluding mutual/index funds). Illumina, Inc. – Employee, Intellectual Property/Patents, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Kristen Petersen: Freenome – Employee, Stock Options, Stock-privately held company.
Mahan Matin: Freenome – Consultant.
Reinier Meester: Freenome Holdings Inc. – Employee.
Lilian Lee: Freenome – Employee.
Aasma Shaukat: Freenome inc – Consultant.
Man Yee Wong, MBBS, MPH, MBA1, Andy Piscitello, 2, Sarah Kinnings, BSc, MSc, PhD3, Kristen Petersen, MS2, Mahan Matin, MD4, Reinier G.S.. Meester, MS, PhD5, Lilian C.. Lee, PhD2, Aasma Shaukat, MD, MPH, FACG6. P0512 - Adenoma Detection Rates from a Large Clinical Validation Study of a Blood-Based Colorectal Cancer Screening Test, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

