Sunday Poster Session
Category: Colorectal Cancer Prevention
P0486 - Multitarget Stool DNA Testing Uptake and Outcomes in an Urban Academic-Community Setting
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Joseph Gunderson, MD
University of Texas Southwestern Medical Center
Dallas, TX
Presenting Author(s)
Joseph Gunderson, MD1, Franklin Liu, MD2, Samuel Cheong, DO3, Alisia C. Gunderson, MD1, Fariba Hossain, BS2, Katherine Mendoza, MPH4, Gloria D. Coronado, PhD4, Richard M.. Hoffman, MD, MPH5
1University of Texas Southwestern Medical Center, Dallas, TX; 2University of Arizona College of Medicine, Tucson, AZ; 3Banner - University of Arizona Tucson, Tucson, AZ; 4University of Arizona, Tucson, AZ; 5University of Iowa Carver College of Medicine, Iowa City, IA
Introduction: The multitarget stool DNA (mt-sDNA) has been approved for colorectal cancer (CRC) screening based on its diagnostic performance characteristics. However, limited data exist on the continuum of implementing mt-sDNA testing in clinical practice, from test completion, positivity rate, referrals for diagnostic colonoscopy (COL), detection of advanced neoplasia (AN) to longitudinal adherence to repeat screening. We described these outcomes for patients undergoing mt-sDNA testing in an urban healthcare system.
Methods: We reviewed patients aged 50-75 with a mt-sDNA test ordered at 16 outpatient sites in a Tucson, AZ healthcare system from 9/9/2017 to 12/31/2021. We collected data on demographics, insurance, CRC screening history, and ordering provider specialty for patients completing testing. Outcomes included test completion, positivity rate, COL referral/completion, AN detection, and rescreening within 40 months of a negative index test. Adjusted logistic regression identified factors linked to rescreening. We limited the rescreening analysis to patients aged 50-72 at initial testing.
Results: 689 (35.8%) of 1,923 patients completed mt-sDNA testing (median age 62 years, 59.5% female, 77.4% White, 14.8% Hispanic or Latino). The median time to test completion was 22 days (IQR 14-38). Insurance included 37.9% private, 41.1% Medicare / Medicare Advantage, and 14.7% Medicaid. Family physicians ordered 65.2% of tests. 32.1% of patients had undergone prior CRC screening.
78 (11.3%) patients tested positive, 573 (83.1%) tested negative, and 38 (5.5%) had an indeterminate result. 74 (94.5%) patients testing positive were referred for COL and 52 (62.3%) completed COL. 21 (40.4%) patients had an AN, including 3 with CRC.
474 of 573 patients testing negative were eligible for 3-year rescreening. By 40-month follow-up, 164 (34.6%) were rescreened: 125 (26.4%) with mt-sDNA, 38 (8.0%) with COL, and 1 (0.2%) with fecal immunohistochemical testing. Only previous CRC screening was linked to improved 40-month rescreening (aOR 1.57, 95% CI 1.01-2.45, P = 0.044).
Discussion: Screening uptake with mt-sDNA was low: 35.8% of patients completed the index test, 62.3% completed COL following a positive test, and 34.6% of eligible patients underwent rescreening within 40 months. Previous screening was associated with 40-month rescreening. These findings raise concerns about the utility of mt-sDNA based-CRC screening programs in clinical practice and warrant further investigation.
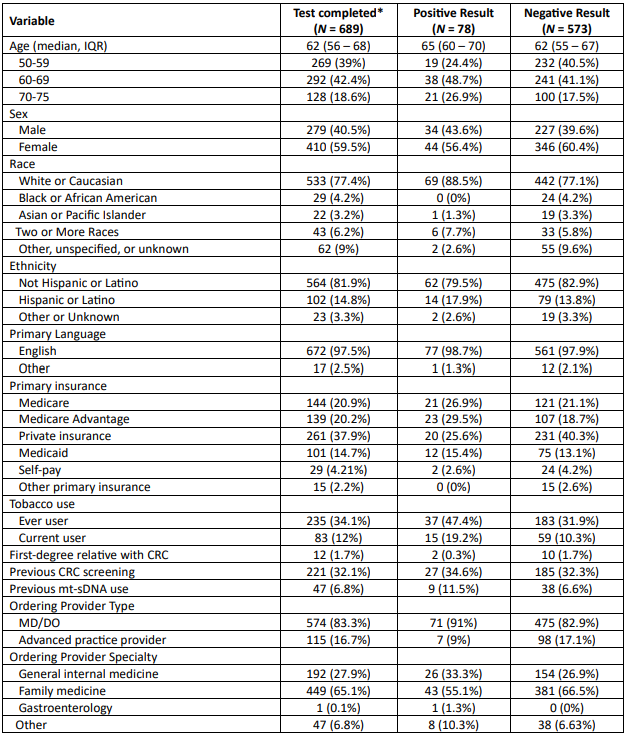
Figure: Table 1. Characteristics and testing outcomes of patients undergoing multitarget stool DNA testing
*38 patients had an indeterminate index result that was not repeated
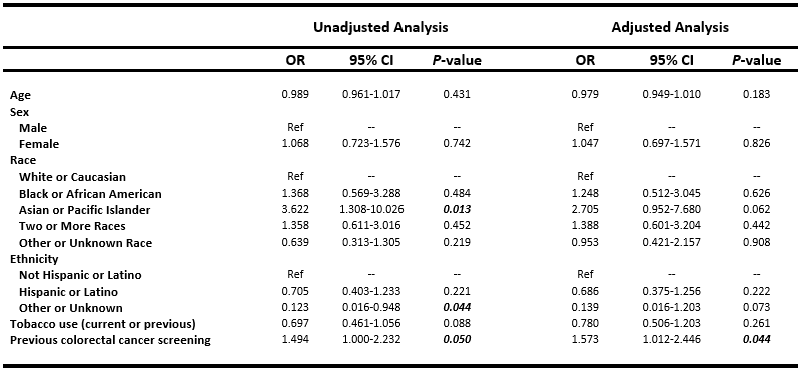
Figure: Table 2. Logistic regression analysis assessing factors associated with 40-month colorectal cancer rescreening among patients undergoing multitarget stool DNA testing
Disclosures:
Joseph Gunderson indicated no relevant financial relationships.
Franklin Liu indicated no relevant financial relationships.
Samuel Cheong indicated no relevant financial relationships.
Alisia Gunderson indicated no relevant financial relationships.
Fariba Hossain indicated no relevant financial relationships.
Katherine Mendoza indicated no relevant financial relationships.
Gloria Coronado indicated no relevant financial relationships.
Richard Hoffman indicated no relevant financial relationships.
Joseph Gunderson, MD1, Franklin Liu, MD2, Samuel Cheong, DO3, Alisia C. Gunderson, MD1, Fariba Hossain, BS2, Katherine Mendoza, MPH4, Gloria D. Coronado, PhD4, Richard M.. Hoffman, MD, MPH5. P0486 - Multitarget Stool DNA Testing Uptake and Outcomes in an Urban Academic-Community Setting, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Texas Southwestern Medical Center, Dallas, TX; 2University of Arizona College of Medicine, Tucson, AZ; 3Banner - University of Arizona Tucson, Tucson, AZ; 4University of Arizona, Tucson, AZ; 5University of Iowa Carver College of Medicine, Iowa City, IA
Introduction: The multitarget stool DNA (mt-sDNA) has been approved for colorectal cancer (CRC) screening based on its diagnostic performance characteristics. However, limited data exist on the continuum of implementing mt-sDNA testing in clinical practice, from test completion, positivity rate, referrals for diagnostic colonoscopy (COL), detection of advanced neoplasia (AN) to longitudinal adherence to repeat screening. We described these outcomes for patients undergoing mt-sDNA testing in an urban healthcare system.
Methods: We reviewed patients aged 50-75 with a mt-sDNA test ordered at 16 outpatient sites in a Tucson, AZ healthcare system from 9/9/2017 to 12/31/2021. We collected data on demographics, insurance, CRC screening history, and ordering provider specialty for patients completing testing. Outcomes included test completion, positivity rate, COL referral/completion, AN detection, and rescreening within 40 months of a negative index test. Adjusted logistic regression identified factors linked to rescreening. We limited the rescreening analysis to patients aged 50-72 at initial testing.
Results: 689 (35.8%) of 1,923 patients completed mt-sDNA testing (median age 62 years, 59.5% female, 77.4% White, 14.8% Hispanic or Latino). The median time to test completion was 22 days (IQR 14-38). Insurance included 37.9% private, 41.1% Medicare / Medicare Advantage, and 14.7% Medicaid. Family physicians ordered 65.2% of tests. 32.1% of patients had undergone prior CRC screening.
78 (11.3%) patients tested positive, 573 (83.1%) tested negative, and 38 (5.5%) had an indeterminate result. 74 (94.5%) patients testing positive were referred for COL and 52 (62.3%) completed COL. 21 (40.4%) patients had an AN, including 3 with CRC.
474 of 573 patients testing negative were eligible for 3-year rescreening. By 40-month follow-up, 164 (34.6%) were rescreened: 125 (26.4%) with mt-sDNA, 38 (8.0%) with COL, and 1 (0.2%) with fecal immunohistochemical testing. Only previous CRC screening was linked to improved 40-month rescreening (aOR 1.57, 95% CI 1.01-2.45, P = 0.044).
Discussion: Screening uptake with mt-sDNA was low: 35.8% of patients completed the index test, 62.3% completed COL following a positive test, and 34.6% of eligible patients underwent rescreening within 40 months. Previous screening was associated with 40-month rescreening. These findings raise concerns about the utility of mt-sDNA based-CRC screening programs in clinical practice and warrant further investigation.

Figure: Table 1. Characteristics and testing outcomes of patients undergoing multitarget stool DNA testing
*38 patients had an indeterminate index result that was not repeated

Figure: Table 2. Logistic regression analysis assessing factors associated with 40-month colorectal cancer rescreening among patients undergoing multitarget stool DNA testing
Disclosures:
Joseph Gunderson indicated no relevant financial relationships.
Franklin Liu indicated no relevant financial relationships.
Samuel Cheong indicated no relevant financial relationships.
Alisia Gunderson indicated no relevant financial relationships.
Fariba Hossain indicated no relevant financial relationships.
Katherine Mendoza indicated no relevant financial relationships.
Gloria Coronado indicated no relevant financial relationships.
Richard Hoffman indicated no relevant financial relationships.
Joseph Gunderson, MD1, Franklin Liu, MD2, Samuel Cheong, DO3, Alisia C. Gunderson, MD1, Fariba Hossain, BS2, Katherine Mendoza, MPH4, Gloria D. Coronado, PhD4, Richard M.. Hoffman, MD, MPH5. P0486 - Multitarget Stool DNA Testing Uptake and Outcomes in an Urban Academic-Community Setting, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
