Sunday Poster Session
Category: Colorectal Cancer Prevention
P0477 - Time to Colonoscopy After Positive Cologuard and Its Association With Colorectal Cancer Stage
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- PB
Puja Brahmbhatt, MD
Loyola University Medical Center
Maywood, IL
Presenting Author(s)
Puja Brahmbhatt, MD1, Michelle Lee, MD1, Vaishnavi K. Modi, MD2, Emma Schnittka, DO1, Kartik Devgan, MD3, Veronica Williams, MD1, Harish Venkataraj, MD1, Amy Wozniak, MS1, Rohit Agrawal, MD1, Ayokunle Abegunde, MD1
1Loyola University Medical Center, Maywood, IL; 2MacNeal Hospital Loyola Medicine, Oak Park, IL; 3Loyola University Medical Center, Chicago, IL
Introduction: Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States. Screening significantly reduces CRC mortality. Multitarget stool DNA (Cologuard) and fecal immunochemical test (FIT) are widely used non-invasive screening options for average-risk adults aged ≥45 years. However, limited data exist on how the time interval from a positive result to diagnostic colonoscopy impacts clinical outcomes, particularly with Cologuard. We evaluated whether time to colonoscopy affects detection of malignancy or high-risk polyps and compared diagnostic yield between Cologuard and FIT.
Methods: We performed a retrospective cohort study of adults aged ≥45 years with a positive Cologuard or FIT at a single academic center between 2016 and 2024. Data included demographics, comorbidities, insurance, medication use, provider type, and colonoscopy findings. The primary outcome was the association between time from positive test to colonoscopy and detection of malignancy or high-risk polyps (≥10 mm, villous features, or high-grade dysplasia). Secondary outcomes included colonoscopy completion rates, diagnostic yield comparison, and predictors of Cologuard positivity. Statistical tests included t-tests, chi-square/Fisher’s exact, and Wilcoxon rank-sum.
Results: Of 1,172 patients (935 Cologuard, 237 FIT), Cologuard users were older (66.1 vs. 62.6 years, p< 0.001), more likely White (73.6% vs. 59.1%, p< 0.001), and had higher rates of hypertension and NSAID use. FIT users had greater antiplatelet use (29.9% vs. 21.4%, p=0.005). Colonoscopy completion rates (64.9% vs. 62.4%, p=0.526) and median time to colonoscopy (2.93 vs. 3.26 months, p=0.961) were similar. Cologuard had higher polyp detection (90.9% vs. 80.4%, p=0.003) and polyp burden (median 3 vs. 2, p=0.009). Cancer detection was comparable (3.5% vs. 4.7%, p=0.491). Anticoagulation or dual antiplatelet therapy was not associated with differences in polyp detection or false-positive rates (p=0.93). Cologuard had higher—but not statistically significant—detection of sessile serrated polyps, high-grade dysplasia, and tubulovillous adenomas.
Discussion: Despite similar follow-up intervals, Cologuard was associated with greater diagnostic yield. These findings support the test’s sensitivity and underscore the importance of timely colonoscopy to optimize detection of advanced neoplasia.
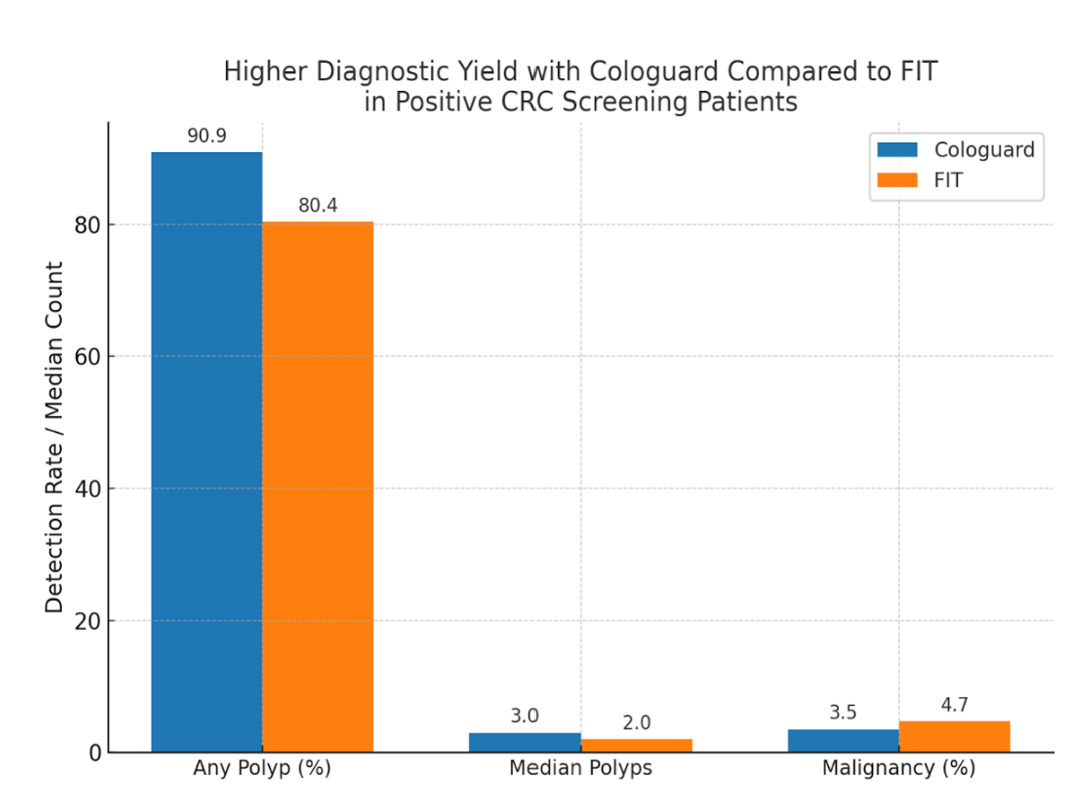
Figure: Figure 1: Cologuard was associated with a higher polyp detection rate (90.9% vs 80.4%, p=0.003) and greater polyp burden (median 3 vs. 2, p=0.009) with similar malignancy rates (3.5% vs 4.7%, p=0.491).
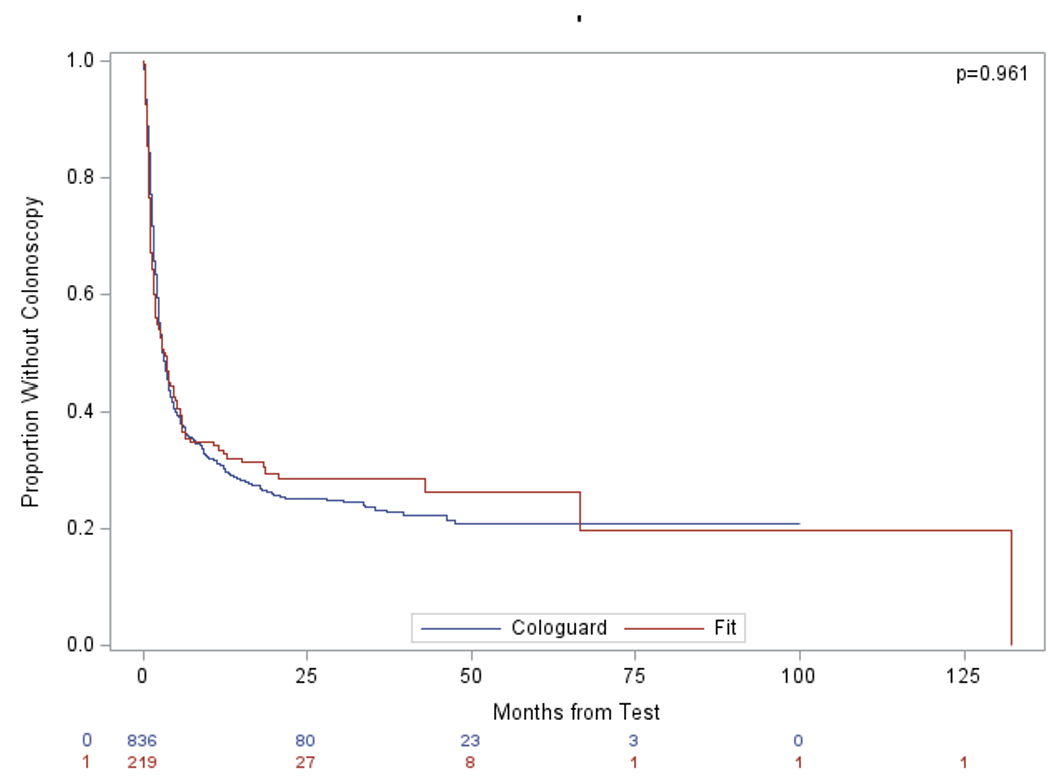
Figure: Figure 2: The median time from test to colonoscopy was as follows: 3.26 months for Fit and 2.93 months for Cologuard, p=0.961.
Disclosures:
Puja Brahmbhatt indicated no relevant financial relationships.
Michelle Lee indicated no relevant financial relationships.
Vaishnavi Modi indicated no relevant financial relationships.
Emma Schnittka indicated no relevant financial relationships.
Kartik Devgan indicated no relevant financial relationships.
Veronica Williams indicated no relevant financial relationships.
Harish Venkataraj indicated no relevant financial relationships.
Amy Wozniak indicated no relevant financial relationships.
Rohit Agrawal indicated no relevant financial relationships.
Ayokunle Abegunde indicated no relevant financial relationships.
Puja Brahmbhatt, MD1, Michelle Lee, MD1, Vaishnavi K. Modi, MD2, Emma Schnittka, DO1, Kartik Devgan, MD3, Veronica Williams, MD1, Harish Venkataraj, MD1, Amy Wozniak, MS1, Rohit Agrawal, MD1, Ayokunle Abegunde, MD1. P0477 - Time to Colonoscopy After Positive Cologuard and Its Association With Colorectal Cancer Stage, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Loyola University Medical Center, Maywood, IL; 2MacNeal Hospital Loyola Medicine, Oak Park, IL; 3Loyola University Medical Center, Chicago, IL
Introduction: Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States. Screening significantly reduces CRC mortality. Multitarget stool DNA (Cologuard) and fecal immunochemical test (FIT) are widely used non-invasive screening options for average-risk adults aged ≥45 years. However, limited data exist on how the time interval from a positive result to diagnostic colonoscopy impacts clinical outcomes, particularly with Cologuard. We evaluated whether time to colonoscopy affects detection of malignancy or high-risk polyps and compared diagnostic yield between Cologuard and FIT.
Methods: We performed a retrospective cohort study of adults aged ≥45 years with a positive Cologuard or FIT at a single academic center between 2016 and 2024. Data included demographics, comorbidities, insurance, medication use, provider type, and colonoscopy findings. The primary outcome was the association between time from positive test to colonoscopy and detection of malignancy or high-risk polyps (≥10 mm, villous features, or high-grade dysplasia). Secondary outcomes included colonoscopy completion rates, diagnostic yield comparison, and predictors of Cologuard positivity. Statistical tests included t-tests, chi-square/Fisher’s exact, and Wilcoxon rank-sum.
Results: Of 1,172 patients (935 Cologuard, 237 FIT), Cologuard users were older (66.1 vs. 62.6 years, p< 0.001), more likely White (73.6% vs. 59.1%, p< 0.001), and had higher rates of hypertension and NSAID use. FIT users had greater antiplatelet use (29.9% vs. 21.4%, p=0.005). Colonoscopy completion rates (64.9% vs. 62.4%, p=0.526) and median time to colonoscopy (2.93 vs. 3.26 months, p=0.961) were similar. Cologuard had higher polyp detection (90.9% vs. 80.4%, p=0.003) and polyp burden (median 3 vs. 2, p=0.009). Cancer detection was comparable (3.5% vs. 4.7%, p=0.491). Anticoagulation or dual antiplatelet therapy was not associated with differences in polyp detection or false-positive rates (p=0.93). Cologuard had higher—but not statistically significant—detection of sessile serrated polyps, high-grade dysplasia, and tubulovillous adenomas.
Discussion: Despite similar follow-up intervals, Cologuard was associated with greater diagnostic yield. These findings support the test’s sensitivity and underscore the importance of timely colonoscopy to optimize detection of advanced neoplasia.

Figure: Figure 1: Cologuard was associated with a higher polyp detection rate (90.9% vs 80.4%, p=0.003) and greater polyp burden (median 3 vs. 2, p=0.009) with similar malignancy rates (3.5% vs 4.7%, p=0.491).

Figure: Figure 2: The median time from test to colonoscopy was as follows: 3.26 months for Fit and 2.93 months for Cologuard, p=0.961.
Disclosures:
Puja Brahmbhatt indicated no relevant financial relationships.
Michelle Lee indicated no relevant financial relationships.
Vaishnavi Modi indicated no relevant financial relationships.
Emma Schnittka indicated no relevant financial relationships.
Kartik Devgan indicated no relevant financial relationships.
Veronica Williams indicated no relevant financial relationships.
Harish Venkataraj indicated no relevant financial relationships.
Amy Wozniak indicated no relevant financial relationships.
Rohit Agrawal indicated no relevant financial relationships.
Ayokunle Abegunde indicated no relevant financial relationships.
Puja Brahmbhatt, MD1, Michelle Lee, MD1, Vaishnavi K. Modi, MD2, Emma Schnittka, DO1, Kartik Devgan, MD3, Veronica Williams, MD1, Harish Venkataraj, MD1, Amy Wozniak, MS1, Rohit Agrawal, MD1, Ayokunle Abegunde, MD1. P0477 - Time to Colonoscopy After Positive Cologuard and Its Association With Colorectal Cancer Stage, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
