Sunday Poster Session
Category: Colon
P0416 - Olmesartan-Induced Enteritis and Microscopic Colitis: A Rare Cause of Chronic Diarrhea
.jpeg.jpg)
Zhexiang He, DO
Conway Regional Health System
Iowa City, IA
Presenting Author(s)
Conway Regional Health System, Conway, AR
Introduction:
Olmesartan, an angiotensin II receptor blocker (ARB), is widely prescribed for managing hypertension. Common side effects include dizziness and hyperglycemia, olmesartan-induced enteropathy is a rare but significant cause of chronic diarrhea, often mimic celiac disease. Until today, no cases of olmesartan-induced microscopic colitis have been reported. Here, we present a rare case of olmesartan-induced enteropathy involving both the small and large bowel.
Case Description/
Methods:
A 73-year-old female presented with a 9-week history of intractable, non-bloody watery diarrhea (9–10 times/day), significant weight loss, and decreased appetite. She denied abdominal pain, nausea, vomiting, bloating, fever, or chills. Her past medical history was unremarkable for GI disease, and she had not recently used antibiotics; family history included celiac disease.
Previously, she was hospitalized at OSH for acute kidney injury and received IV fluids without further workup. On admission to our facility, labs showed leukocytosis (WBC 21,000/μL), mild lactic acidosis, hypokalemia, hypomagnesemia, hemoglobin drop (15.6 to 11.6 g/dL), elevated lipase (220 U/L), and normal LFTs. CT scan suggested enteritis; stool studies were negative.
Further evaluation showed 20% fecal fat, low pancreatic elastase (59 μg/g), negative celiac serologies, and normal colonoscopy, but biopsies confirmed microscopic colitis. Given the recent initiation of olmesartan, the drug was identified as the likely cause.
Olmesartan was discontinued, and treatment with rifaximin, cholestyramine, and a liquid diet led to full recovery. At one-month follow-up, she reported complete symptom resolution.
Discussion:
Olmesartan-induced enteropathy, first described in 2012, can mimic celiac disease or inflammatory bowel disease, manifesting as chronic diarrhea, weight loss, and malabsorption. Diagnosis is largely clinical and relies on recognizing the characteristic pattern of symptom resolution after discontinuing the medication.
While olmesartan-induced enteropathy is well documented, reports of associated microscopic colitis are lacking, making this case particularly noteworthy.
This case highlights the importance of considering drug-induced enteropathies in patients with unexplained chronic diarrhea, particularly among older adults or those with recent changes in medication. Timely recognition and withdrawal of the offending agent can prevent unnecessary invasive testing, reduce morbidity, and facilitate recovery.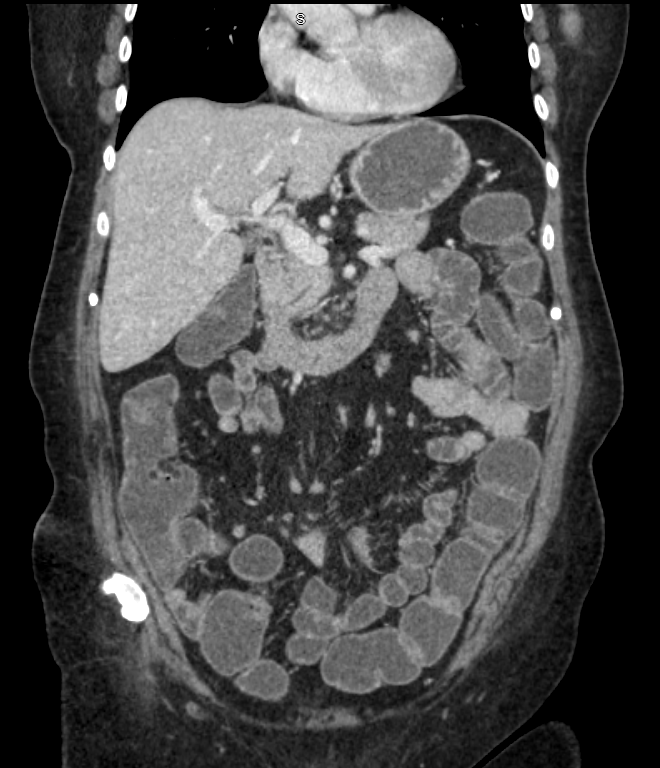
Figure: Abdominal/Pelvic CT shows fluid and gas filled in the small bowel loop, compatible with enteritis. 
Figure: Pathology shows increased plasma cells in the lamina propria and scattered lymphocytes in the glands and limited Subepithelial collagen deposition - findings are typical for microscopic colitis
Disclosures:
Zhexiang He indicated no relevant financial relationships.
Manasa Ranginani indicated no relevant financial relationships.
Anastasia Almyasheva indicated no relevant financial relationships.
Shuja Khan indicated no relevant financial relationships.
Zhexiang He, DO, Manasa Ranginani, MD, Anastasia Almyasheva, DO, Shuja Khan, MD. P0416 - Olmesartan-Induced Enteritis and Microscopic Colitis: A Rare Cause of Chronic Diarrhea, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
