Sunday Poster Session
Category: Colon
P0316 - Demographic Variations in Immune Checkpoint Inhibitor Associated Gastrointestinal Adverse Effects – Real World Study
- SS
Shreya Shambhavi, MD (she/her/hers)
Rutgers Health/Community Medical Center
Toms River, NJ
Presenting Author(s)
1Rutgers Health/Community Medical Center, Toms River, NJ; 2Mary Washington Healthcare, Fredericksburg, VA; 3Mahatma Gandhi Memorial Medical College, Jamshedpur, White Rock, BC, Canada; 4Saint Clare's Health, New York Medical College, Denville, NJ
Introduction:
Immune checkpoint inhibitors (ICIs) have transformed cancer therapy but can trigger immune-related adverse events (irAEs). Immune-mediated colitis (IMC) affects 5.7 %–39.1 % of CTLA-4 inhibitor recipients and 0.7 %–31.6 % of PD-1/PD-L1 inhibitor recipients, rising to 40.4 % with combination therapy. CTLA-4 monotherapy carries up to a 15 % risk of immune-mediated hepatitis (IMH), whereas PD-1/PD-L1 monotherapy carries ≤ 6 %; combining CTLA-4 with PD-1 roughly doubles to triples IMH rates (≈ 13 %–15 %). Yet detailed analyses of irAE variation by BMI, race, sex, age, and ICI type remain scarce.
Methods:
A retrospective analysis was conducted on 244 patients who developed irAEs after receiving ICIs. Univariate analysis was performed employing the Chi-square test. Multivariable logistic regression and cluster analyses revealed distinct irAE predictors.
Results:
Univariate analysis showed a possible association between race and hepatitis (χ² = 8.18, df = 3, p = 0.04), but this was not confirmed by multivariable logistic regression after adjusting for confounders. Cluster analysis found higher hepatitis frequency in pembrolizumab monotherapy patients versus those on nivolumab or nivolumab + ipilimumab (67.6 % vs. 63.8 %). For colitis, neither univariate nor multivariable analyses revealed significant links with sex, race, BMI, or age. Cluster analysis showed higher colitis incidence in the nivolumab or nivolumab + ipilimumab clusters compared to the pembrolizumab cluster (84.5 % vs. 72.7 %).
Discussion:
Although univariate analysis hinted at a relationship between race and the development of immune‐mediated hepatitis, this association did not hold after adjusting for potential confounders in multivariable modeling. No demographic or clinical factors, including sex, race, BMI, or age were significantly linked to immune‐mediated colitis in either univariate or multivariable analyses. However, cluster analysis suggests that colitis is more frequent among patients receiving nivolumab (alone or in combination with ipilimumab) compared with those receiving pembrolizumab (84.5 % vs. 72.7 %). These findings imply that, aside from the specific checkpoint‐inhibitor regimen, traditional demographic risk factors may have limited predictive value for colitis, and that race may not independently predict hepatitis once other variables are accounted for.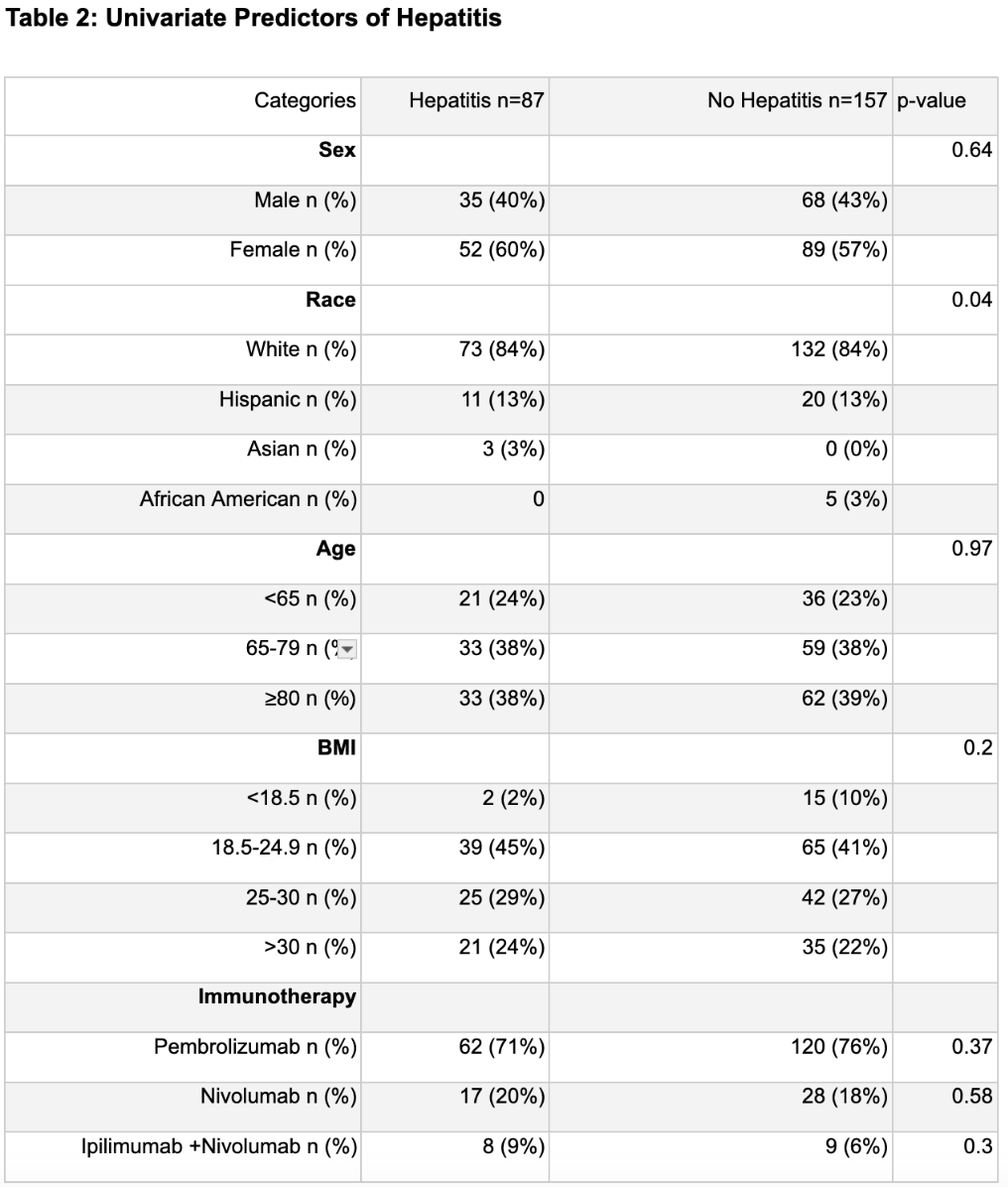
Figure: Univariate Predictors of Hepatitis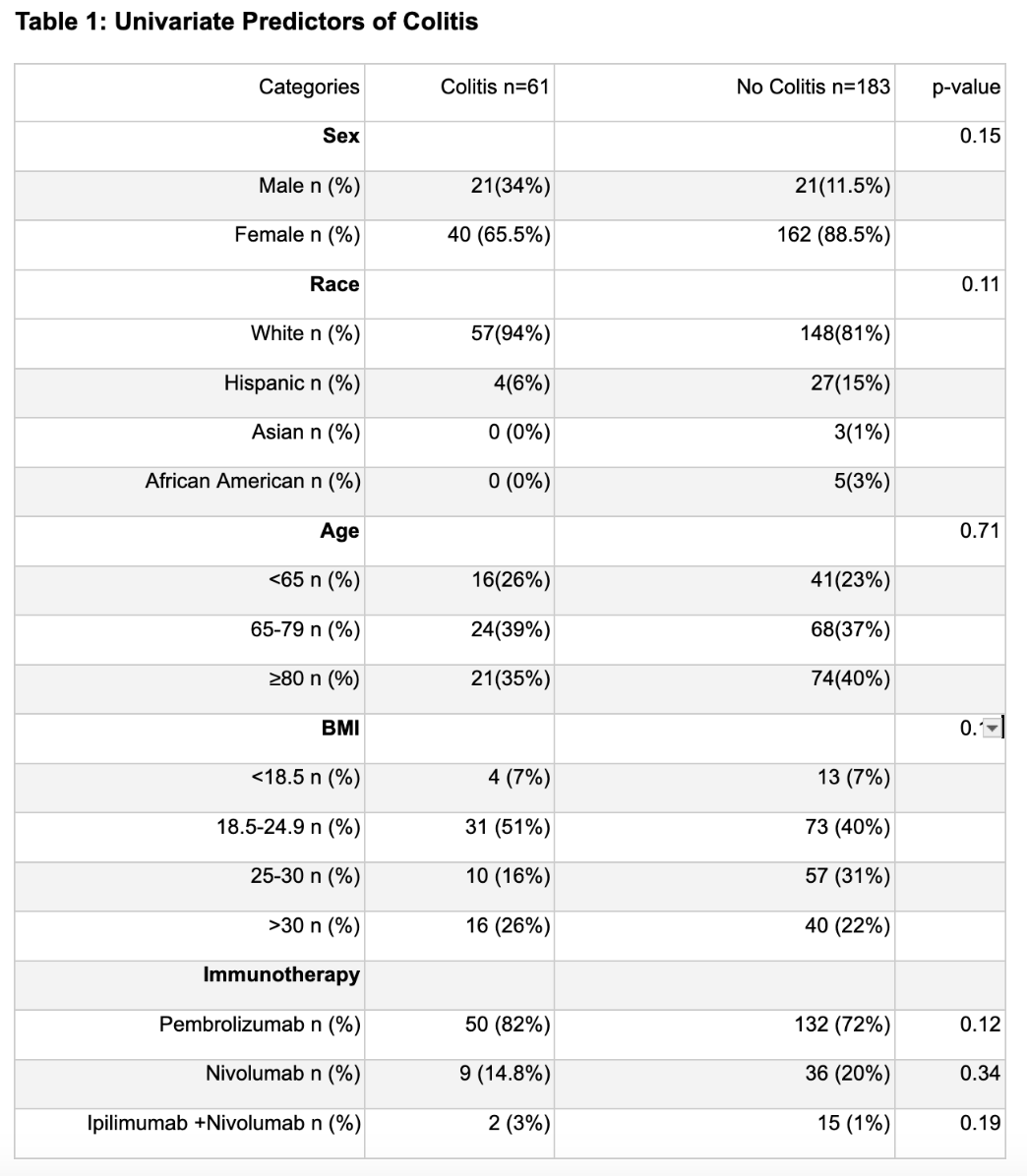
Figure: Univariate Predictors of Colitis
Disclosures:
Shreya Shambhavi indicated no relevant financial relationships.
Ganesh Ramaprasad indicated no relevant financial relationships.
Harmanjeet Singh indicated no relevant financial relationships.
Vinod Nookala indicated no relevant financial relationships.
Shreya Shambhavi, MD1, Ganesh Ramaprasad, MD2, Harmanjeet Singh, MBBS3, Vinod Nookala, MD4. P0316 - Demographic Variations in Immune Checkpoint Inhibitor Associated Gastrointestinal Adverse Effects – Real World Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
