Sunday Poster Session
Category: Colon
P0263 - Accelerated vs Standard Infliximab Induction in Acute Severe Ulcerative Colitis: An Updated Meta-Analysis of Colectomy Outcomes
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- RT
Rukshar Thapa, MD (she/her/hers)
Lankenau Medical Center
Penn Wynne, PA
Presenting Author(s)
Munna William, MBBS1, Rukshar Thapa, MD2, Momena Rashid, MBBS1, Nirmal Paudel, 1, Anurag Jha, 3, Pragyat Singh, MBBS4, Venkateswarlu Chintagumpala, MD5
1Nishtar Medical University, Multan, Punjab, Pakistan; 2Lankenau Medical Center, Penn Wynne, PA; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4Nepalese Army Institute of Health Sciences, Kathmandu, Bagmati, Nepal; 5Drexel University, Philadelphia, PA
Introduction: Acute severe ulcerative colitis (ASUC) often requires infliximab in patients unresponsive to steroids. While accelerated induction regimens aim to improve outcomes by overcoming rapid drug clearance, their benefit over standard induction remains uncertain. This meta-analysis compares colectomy outcomes between accelerated and standard infliximab induction in ASUC.
Methods: A comprehensive literature search of 4 electronic databases was conducted from inception to 15 April 2025 for the study comparing accelerated (defined as infliximab doses administered at weeks 0, 1, and 2 or similar intensified schedules) versus standard (0, 2, and 6 weeks) infliximab induction in ASUC patients. The outcomes assessed include overall and 3-month colectomy rates. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using a random-effects model. Heterogeneity was assessed using the I² statistic.
Results: A total of eight studies involving 833 patients (294 in the accelerated group and 539 in the standard group) were included for analysis of overall colectomy. The pooled odds ratio was 1.03 (95% CI: 0.54–1.94), indicating no statistically significant difference in overall colectomy rates between the two groups (P = 0.94). There was moderate heterogeneity across studies (I² = 60%), and the overall effect estimate was close to null (Figure 1). In the 3-month colectomy subgroup analysis (5 studies, 166 in the accelerated group vs. 269 in the standard group), the pooled odds ratio was 1.69 (95% CI: 0.67–4.25), again showing no statistically significant difference (P = 0.27). However, there was a trend toward reduced colectomy with accelerated induction. Heterogeneity remained moderate (I² = 59%). Individual study results varied, with one study (Liu 2024) reporting a notably high OR favoring accelerated induction (OR: 12.70), contributing to wider confidence intervals and increased variance (Figure 2).
Discussion: Accelerated infliximab induction did not significantly reduce overall or 3-month colectomy rates compared to standard induction in ASUC patients. While a trend toward benefit was seen at 3 months, heterogeneity and wide confidence intervals limit definitive conclusions. Further high-quality studies are warranted.
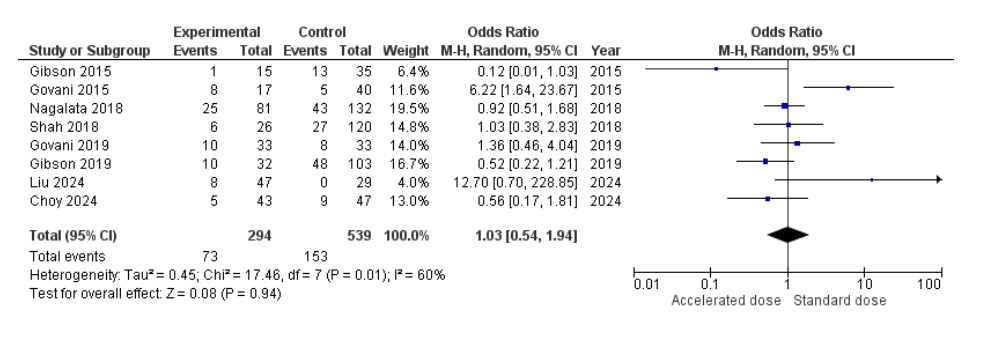
Figure: Figure 1: Forest plot comparing overall colectomy rate between accelerated and standard infliximab induction therapy
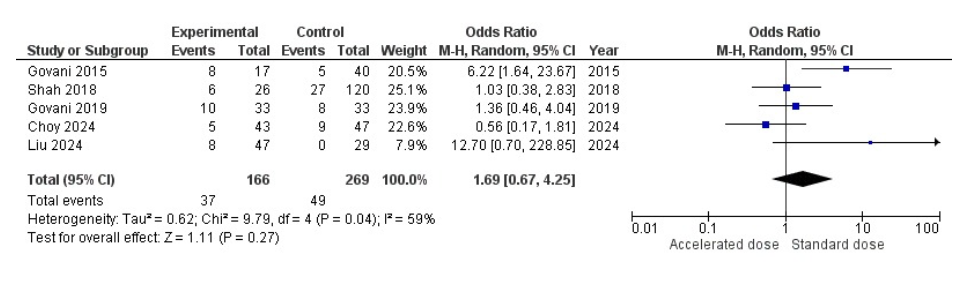
Figure: Figure 2: Forest plot comparing 3-month colectomy rate between accelerated and standard infliximab induction therapy
Disclosures:
Munna William indicated no relevant financial relationships.
Rukshar Thapa indicated no relevant financial relationships.
Momena Rashid indicated no relevant financial relationships.
Nirmal Paudel indicated no relevant financial relationships.
Anurag Jha indicated no relevant financial relationships.
Pragyat Singh indicated no relevant financial relationships.
Venkateswarlu Chintagumpala indicated no relevant financial relationships.
Munna William, MBBS1, Rukshar Thapa, MD2, Momena Rashid, MBBS1, Nirmal Paudel, 1, Anurag Jha, 3, Pragyat Singh, MBBS4, Venkateswarlu Chintagumpala, MD5. P0263 - Accelerated vs Standard Infliximab Induction in Acute Severe Ulcerative Colitis: An Updated Meta-Analysis of Colectomy Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Nishtar Medical University, Multan, Punjab, Pakistan; 2Lankenau Medical Center, Penn Wynne, PA; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4Nepalese Army Institute of Health Sciences, Kathmandu, Bagmati, Nepal; 5Drexel University, Philadelphia, PA
Introduction: Acute severe ulcerative colitis (ASUC) often requires infliximab in patients unresponsive to steroids. While accelerated induction regimens aim to improve outcomes by overcoming rapid drug clearance, their benefit over standard induction remains uncertain. This meta-analysis compares colectomy outcomes between accelerated and standard infliximab induction in ASUC.
Methods: A comprehensive literature search of 4 electronic databases was conducted from inception to 15 April 2025 for the study comparing accelerated (defined as infliximab doses administered at weeks 0, 1, and 2 or similar intensified schedules) versus standard (0, 2, and 6 weeks) infliximab induction in ASUC patients. The outcomes assessed include overall and 3-month colectomy rates. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using a random-effects model. Heterogeneity was assessed using the I² statistic.
Results: A total of eight studies involving 833 patients (294 in the accelerated group and 539 in the standard group) were included for analysis of overall colectomy. The pooled odds ratio was 1.03 (95% CI: 0.54–1.94), indicating no statistically significant difference in overall colectomy rates between the two groups (P = 0.94). There was moderate heterogeneity across studies (I² = 60%), and the overall effect estimate was close to null (Figure 1). In the 3-month colectomy subgroup analysis (5 studies, 166 in the accelerated group vs. 269 in the standard group), the pooled odds ratio was 1.69 (95% CI: 0.67–4.25), again showing no statistically significant difference (P = 0.27). However, there was a trend toward reduced colectomy with accelerated induction. Heterogeneity remained moderate (I² = 59%). Individual study results varied, with one study (Liu 2024) reporting a notably high OR favoring accelerated induction (OR: 12.70), contributing to wider confidence intervals and increased variance (Figure 2).
Discussion: Accelerated infliximab induction did not significantly reduce overall or 3-month colectomy rates compared to standard induction in ASUC patients. While a trend toward benefit was seen at 3 months, heterogeneity and wide confidence intervals limit definitive conclusions. Further high-quality studies are warranted.

Figure: Figure 1: Forest plot comparing overall colectomy rate between accelerated and standard infliximab induction therapy

Figure: Figure 2: Forest plot comparing 3-month colectomy rate between accelerated and standard infliximab induction therapy
Disclosures:
Munna William indicated no relevant financial relationships.
Rukshar Thapa indicated no relevant financial relationships.
Momena Rashid indicated no relevant financial relationships.
Nirmal Paudel indicated no relevant financial relationships.
Anurag Jha indicated no relevant financial relationships.
Pragyat Singh indicated no relevant financial relationships.
Venkateswarlu Chintagumpala indicated no relevant financial relationships.
Munna William, MBBS1, Rukshar Thapa, MD2, Momena Rashid, MBBS1, Nirmal Paudel, 1, Anurag Jha, 3, Pragyat Singh, MBBS4, Venkateswarlu Chintagumpala, MD5. P0263 - Accelerated vs Standard Infliximab Induction in Acute Severe Ulcerative Colitis: An Updated Meta-Analysis of Colectomy Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
