Sunday Poster Session
Category: Biliary/Pancreas
P0202 - A Complex Case of Pancreaticopleural Fistula: Challenges in Diagnosis and Multidisciplinary Management
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Adarsh Kumar Jha, MD
University of Michigan Health - Sparrow
Lansing, MI
Presenting Author(s)
Adarsh Kumar. Jha, MD1, Amey Joshi, MD1, Divij K. Jha, MD1, Samiksha Pandey, MD2, Shreeya kumar. Adhikari, MD1, Maitri Shah, MD1
1University of Michigan Health - Sparrow, Lansing, MI; 2Corewellhealth William Beaumont University Hospital, Royal Oak, MI
Introduction: Pancreaticopleural fistula (PPF) represents a rare yet formidable complication of chronic pancreatitis, resulting from a pathological communication between the pancreatic ductal system and the pleural cavity. Accounting for approximately 0.4% of all pancreatitis cases, PPF often arises from longstanding ductal inflammation and disruption. Enzymatic secretions leak into the thoracic cavity, leading to massive, recurrent pleural effusions that frequently masquerade as primary pulmonary disease. Clinical presentation is dominated by pulmonary symptoms such as dyspnea (76%), chest pain (30%), and cough (22%), often in the absence of abdominal signs, contributing to an average diagnostic delay of five weeks. Markedly elevated pleural fluid amylase remains a vital diagnostic clue. Early recognition is critical, as delayed diagnosis can result in life-threatening complications.
Case Description/
Methods: A 36-year-old woman with a history of recurrent acute-on-chronic pancreatitis presented with acute respiratory distress requiring 12L oxygen. Imaging revealed a large left loculated pleural effusion, mediastinal shift, and multiple pancreatic pseudocysts. Thoracentesis drained 300 mL of exudative fluid with WBC 5000/μL, LDH 3136 IU/L, protein 7 g/dL, and amylase 5137 U/L. ERCP identified a ductal leak in the distal pancreatic body/tail. A 7 Fr × 12 cm stent was placed; however, it was found to not have traversed the tail. The course was complicated by empyema thoracis, hydropneumothorax, and fibrosis requiring VATS with decortication and fibrinolysis. Follow-up CT showed stable necrotic collections. As her condition improved, distal pancreatectomy was deferred in favor of conservative management with nasojejunal feeding and medical therapy.
Discussion: PPF most commonly results from posterior pseudocyst rupture or ductal disruption, with pancreatic secretions entering the pleural space via diaphragmatic pathways. Diagnosis is often delayed due to predominant thoracic symptoms. Investigations include MRCP (80% sensitivity), ERCP (78%), and CT (47%). Management begins bowel rest, octreotide, TPN, and pleural drainage. Stenting is successful in 70–82% of cases. Surgery is reserved for ductal disruption in the tail or failed ERCP, with success rates up to 94%. Clinicians must maintain high suspicion for PPF in patients with unexplained pleural effusions and a history of pancreatitis. Elevated pleural amylase should prompt urgent evaluation. Early diagnosis and multidisciplinary intervention are key.
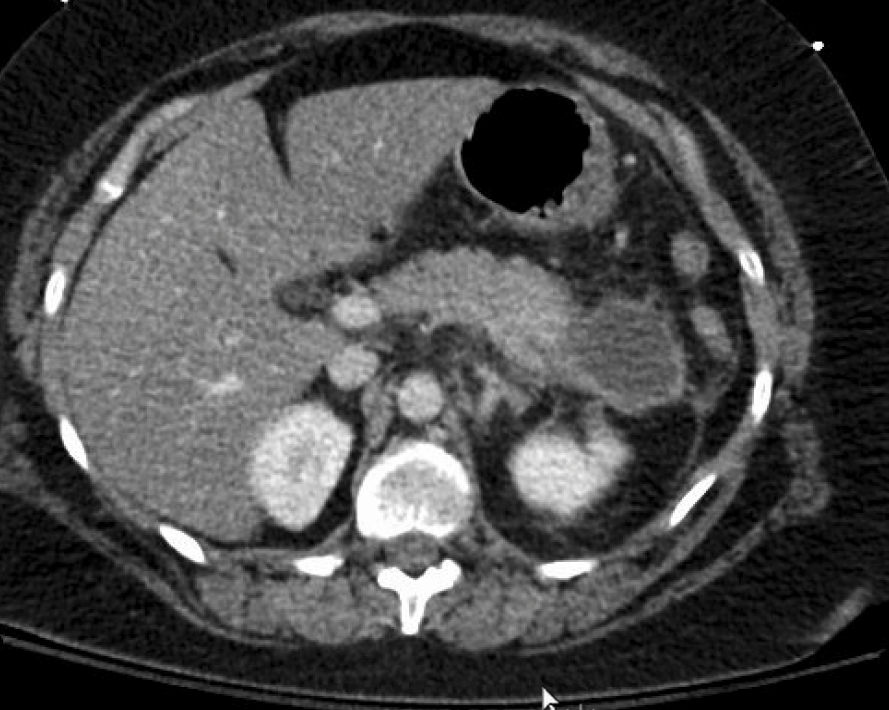
Figure: Fig 1: Multiple cystic structures in the LEFT upper quadrant, representing pancreatic pseudocysts. Largest, in the tail region of the pancreas, measures about 4.5 x 4.0 cm.
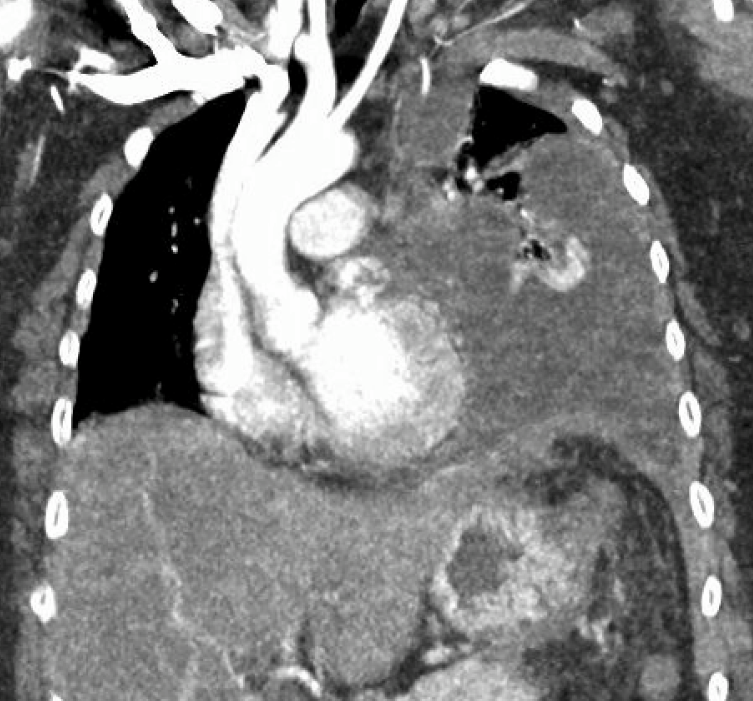
Figure: Fig 2: Interval development of a large loculated LEFT-sided pleural effusion with subtotal atelectasis of the LEFT lung. Shift of the cardiomediastinal structures toward the RIGHT. Interval development of multiple cystic structures in the LEFT upper quadrant likely to represent pancreatic pseudocysts.
Disclosures:
Adarsh Jha indicated no relevant financial relationships.
Amey Joshi indicated no relevant financial relationships.
Divij Jha indicated no relevant financial relationships.
Samiksha Pandey indicated no relevant financial relationships.
Shreeya Adhikari indicated no relevant financial relationships.
Maitri Shah indicated no relevant financial relationships.
Adarsh Kumar. Jha, MD1, Amey Joshi, MD1, Divij K. Jha, MD1, Samiksha Pandey, MD2, Shreeya kumar. Adhikari, MD1, Maitri Shah, MD1. P0202 - A Complex Case of Pancreaticopleural Fistula: Challenges in Diagnosis and Multidisciplinary Management, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Michigan Health - Sparrow, Lansing, MI; 2Corewellhealth William Beaumont University Hospital, Royal Oak, MI
Introduction: Pancreaticopleural fistula (PPF) represents a rare yet formidable complication of chronic pancreatitis, resulting from a pathological communication between the pancreatic ductal system and the pleural cavity. Accounting for approximately 0.4% of all pancreatitis cases, PPF often arises from longstanding ductal inflammation and disruption. Enzymatic secretions leak into the thoracic cavity, leading to massive, recurrent pleural effusions that frequently masquerade as primary pulmonary disease. Clinical presentation is dominated by pulmonary symptoms such as dyspnea (76%), chest pain (30%), and cough (22%), often in the absence of abdominal signs, contributing to an average diagnostic delay of five weeks. Markedly elevated pleural fluid amylase remains a vital diagnostic clue. Early recognition is critical, as delayed diagnosis can result in life-threatening complications.
Case Description/
Methods: A 36-year-old woman with a history of recurrent acute-on-chronic pancreatitis presented with acute respiratory distress requiring 12L oxygen. Imaging revealed a large left loculated pleural effusion, mediastinal shift, and multiple pancreatic pseudocysts. Thoracentesis drained 300 mL of exudative fluid with WBC 5000/μL, LDH 3136 IU/L, protein 7 g/dL, and amylase 5137 U/L. ERCP identified a ductal leak in the distal pancreatic body/tail. A 7 Fr × 12 cm stent was placed; however, it was found to not have traversed the tail. The course was complicated by empyema thoracis, hydropneumothorax, and fibrosis requiring VATS with decortication and fibrinolysis. Follow-up CT showed stable necrotic collections. As her condition improved, distal pancreatectomy was deferred in favor of conservative management with nasojejunal feeding and medical therapy.
Discussion: PPF most commonly results from posterior pseudocyst rupture or ductal disruption, with pancreatic secretions entering the pleural space via diaphragmatic pathways. Diagnosis is often delayed due to predominant thoracic symptoms. Investigations include MRCP (80% sensitivity), ERCP (78%), and CT (47%). Management begins bowel rest, octreotide, TPN, and pleural drainage. Stenting is successful in 70–82% of cases. Surgery is reserved for ductal disruption in the tail or failed ERCP, with success rates up to 94%. Clinicians must maintain high suspicion for PPF in patients with unexplained pleural effusions and a history of pancreatitis. Elevated pleural amylase should prompt urgent evaluation. Early diagnosis and multidisciplinary intervention are key.

Figure: Fig 1: Multiple cystic structures in the LEFT upper quadrant, representing pancreatic pseudocysts. Largest, in the tail region of the pancreas, measures about 4.5 x 4.0 cm.

Figure: Fig 2: Interval development of a large loculated LEFT-sided pleural effusion with subtotal atelectasis of the LEFT lung. Shift of the cardiomediastinal structures toward the RIGHT. Interval development of multiple cystic structures in the LEFT upper quadrant likely to represent pancreatic pseudocysts.
Disclosures:
Adarsh Jha indicated no relevant financial relationships.
Amey Joshi indicated no relevant financial relationships.
Divij Jha indicated no relevant financial relationships.
Samiksha Pandey indicated no relevant financial relationships.
Shreeya Adhikari indicated no relevant financial relationships.
Maitri Shah indicated no relevant financial relationships.
Adarsh Kumar. Jha, MD1, Amey Joshi, MD1, Divij K. Jha, MD1, Samiksha Pandey, MD2, Shreeya kumar. Adhikari, MD1, Maitri Shah, MD1. P0202 - A Complex Case of Pancreaticopleural Fistula: Challenges in Diagnosis and Multidisciplinary Management, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
