Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 1A: Infection, Microbiome, Small Intestine
14 - Risks of Gastrointestinal Malignancies in Short Bowel Syndrome Patients Treated With Teduglutide: Results From TriNetX
Monday, October 27, 2025
2:45 PM - 2:55 PM PDT
Location: North Ballroom 120D

Nurlan Aliyev, MD
University of Nebraska Medical Center
Elkhorn, NE
Presenting Author(s)
Award: ACG Outstanding Research Award in the Small Intestine Category
Nurlan Aliyev, MD1, Anasua Deb, MD2, Bilal Niazi, MD2, Ahmad Nawaz, MBBS3, Faruq Pradhan, MBBCh2, Fedja A. Rochling, MBBCh, MBA4
1University of Nebraska Medical Center, Elkhorn, NE; 2University of Nebraska Medical Center, Omaha, NE; 3SUNY Upstate Medical University, Syracuse, NY; 4UNMC, Omaha, NE
Introduction: Teduglutide, a recombinant glucagon-like peptide-2 analog, enhances intestinal absorption and is approved for treating short bowel syndrome (SBS). The STEPS trial reported a treatment-related case of metastatic gastrointestinal (GI) adenocarcinoma, raising concerns about malignancy risk with teduglutide. We aimed to evaluate the incidence of GI malignancies—specifically esophageal, pancreatic, small bowel, and colon—in SBS patients treated with teduglutide using real-world data from TriNetX.
Methods: We performed a retrospective cohort study using the TriNetX Global Collaborative Network. Two cohorts were identified: SBS patients treated with teduglutide (n=514), and control (SBS patients not treated with teduglutide) (n=12,120). Patients with a prior history of esophageal, gastric, pancreatic, small bowel, or colon cancer were excluded. After 1:1 propensity score matching, 514 patients remained in each group. Cancer incidence was assessed 180 days post-index. Outcomes included the incidence rate, risk differences, and esophageal, gastric, pancreatic, and colon cancers. Analyses included risk comparisons and Kaplan-Meier survival.
Results: Post-matching, cohorts were balanced for demographics and comorbidities. Mean follow-up time was 950 days in the teduglutide group vs. 1,219 days in the control group.
Discussion: Despite earlier concerns, this large, matched cohort study found no increased incidence of GI malignancies in SBS patients treated with teduglutide. Notably, colon and esophageal cancers were observed exclusively in the control group. These findings support the long-term oncologic safety of teduglutide in SBS patients.
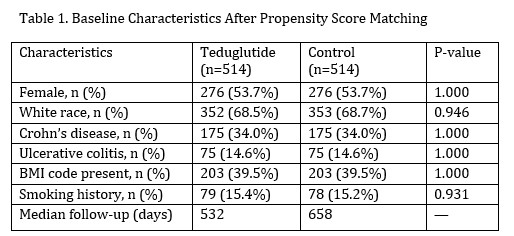
Figure: Baseline characteristic
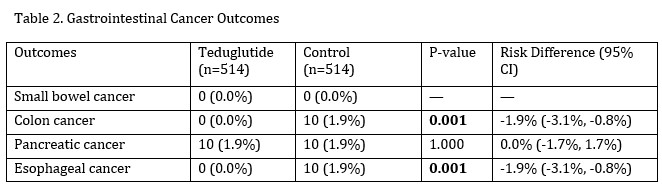
Figure: Outcomes
Disclosures:
Nurlan Aliyev indicated no relevant financial relationships.
Anasua Deb indicated no relevant financial relationships.
Bilal Niazi indicated no relevant financial relationships.
Ahmad Nawaz indicated no relevant financial relationships.
Faruq Pradhan: Ipsen Biopharmaceuticals – Advisory Committee/Board Member. Madrigal Pharmaceuticals – Advisory Committee/Board Member.
Fedja Rochling indicated no relevant financial relationships.
Nurlan Aliyev, MD1, Anasua Deb, MD2, Bilal Niazi, MD2, Ahmad Nawaz, MBBS3, Faruq Pradhan, MBBCh2, Fedja A. Rochling, MBBCh, MBA4, 14, Risks of Gastrointestinal Malignancies in Short Bowel Syndrome Patients Treated With Teduglutide: Results From TriNetX, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Nurlan Aliyev, MD1, Anasua Deb, MD2, Bilal Niazi, MD2, Ahmad Nawaz, MBBS3, Faruq Pradhan, MBBCh2, Fedja A. Rochling, MBBCh, MBA4
1University of Nebraska Medical Center, Elkhorn, NE; 2University of Nebraska Medical Center, Omaha, NE; 3SUNY Upstate Medical University, Syracuse, NY; 4UNMC, Omaha, NE
Introduction: Teduglutide, a recombinant glucagon-like peptide-2 analog, enhances intestinal absorption and is approved for treating short bowel syndrome (SBS). The STEPS trial reported a treatment-related case of metastatic gastrointestinal (GI) adenocarcinoma, raising concerns about malignancy risk with teduglutide. We aimed to evaluate the incidence of GI malignancies—specifically esophageal, pancreatic, small bowel, and colon—in SBS patients treated with teduglutide using real-world data from TriNetX.
Methods: We performed a retrospective cohort study using the TriNetX Global Collaborative Network. Two cohorts were identified: SBS patients treated with teduglutide (n=514), and control (SBS patients not treated with teduglutide) (n=12,120). Patients with a prior history of esophageal, gastric, pancreatic, small bowel, or colon cancer were excluded. After 1:1 propensity score matching, 514 patients remained in each group. Cancer incidence was assessed 180 days post-index. Outcomes included the incidence rate, risk differences, and esophageal, gastric, pancreatic, and colon cancers. Analyses included risk comparisons and Kaplan-Meier survival.
Results: Post-matching, cohorts were balanced for demographics and comorbidities. Mean follow-up time was 950 days in the teduglutide group vs. 1,219 days in the control group.
- Small Bowel Cancer: No cases in either group.
- Colon Cancer: 0 cases in the teduglutide group vs. 10 cases (1.9%) in controls (Risk difference: -0.019; 95% CI: -0.031 to -0.008; p = 0.001). Kaplan-Meier survival analysis favored teduglutide (p = 0.002).
- Pancreatic Cancer: 10 cases (1.9%) in each group; no significant difference (Risk difference = 0; p = 1.0).
- Esophageal Cancer: 0 cases in the teduglutide group vs 10 cases (1.9%) in controls (Risk difference = -0.019; p = 0.001). Survival analysis was not statistically significant (p = 0.314).
Discussion: Despite earlier concerns, this large, matched cohort study found no increased incidence of GI malignancies in SBS patients treated with teduglutide. Notably, colon and esophageal cancers were observed exclusively in the control group. These findings support the long-term oncologic safety of teduglutide in SBS patients.

Figure: Baseline characteristic

Figure: Outcomes
Disclosures:
Nurlan Aliyev indicated no relevant financial relationships.
Anasua Deb indicated no relevant financial relationships.
Bilal Niazi indicated no relevant financial relationships.
Ahmad Nawaz indicated no relevant financial relationships.
Faruq Pradhan: Ipsen Biopharmaceuticals – Advisory Committee/Board Member. Madrigal Pharmaceuticals – Advisory Committee/Board Member.
Fedja Rochling indicated no relevant financial relationships.
Nurlan Aliyev, MD1, Anasua Deb, MD2, Bilal Niazi, MD2, Ahmad Nawaz, MBBS3, Faruq Pradhan, MBBCh2, Fedja A. Rochling, MBBCh, MBA4, 14, Risks of Gastrointestinal Malignancies in Short Bowel Syndrome Patients Treated With Teduglutide: Results From TriNetX, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.



