Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 1B: Pancreas
16 - Characterizing Treatment Satisfaction and Health-Related Quality of Life of Patients With Exocrine Pancreatic Insufficiency (EPI) Taking Pancreatic Enzyme Replacement Therapy (PERT): Findings From the PACT-CP Registry
Monday, October 27, 2025
2:25 PM - 2:35 PM PDT
Location: North Ballroom 120BC
.jpg)
Jodie A. Barkin, MD, FACG
Associate Professor of Clinical Medicine
University of Miami Miller School of Medicine
Miami, FL
Presenting Author(s)
Award: ACG Outstanding Research Award in the Biliary/Pancreas Category
Jodie A. Barkin, MD, FACG1, Ismail Aouadi, MStat2, Jane Diamond, MS, FNP-BC3, Janine Twal, PharmD2, Jennifer Pack, MSN, ACNP-BC2, Trudi Delk, PharmD2, Cindy Frommor, BS4, Dianne Nguyen, MD2, David C. Whitcomb, MD, PhD5
1University of Miami Miller School of Medicine, Miami, FL; 2Nestlé Health Science, Bridgewater, NJ; 3Gastroenterology Department, Mayo Clinic, Jacksonville, FL; 4PPD Observational Studies, Thermo Fisher Scientific, Waltham, MA; 5Department of Medicine, Cell Biology & Physiology and Human Genetics, Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh, UPMC and Ariel Precision Medicine, Pittsburgh, PA
Introduction: PERT is the mainstay of EPI treatment. Frequently, patients (pts) receiving PERT may have multiple comorbidities requiring polypharmacy, which can contribute to pill burden, influence treatment satisfaction, impact PERT adherence, and negatively affect health-related quality of life (HRQoL). The aim of this study was to assess these factors or aspects in pts with EPI with chronic pancreatitis (CP) or recurrent acute pancreatitis (RAP).
Methods: The US-based PAtient-CenTric Chronic Pancreatitis (PACT-CP) registry (NCT05762445) is a prospective, pt-driven registry designed to understand the unmet needs/burden of pts with EPI. Data were obtained from adults with suspected/confirmed EPI, a CP/RAP diagnosis, and taking PERT. Clinical characteristics, PERT use, treatment satisfaction (using the Treatment Satisfaction Questionnaire for Medication-9), and HRQoL (using physical [PCS-12] and mental [MCS-12] health scores) were collected from pts and/or their healthcare providers. Data from baseline to 6 months in pts receiving PERT (pancrelipase, Zenpep® Delayed-Release Capsules) are reported.
Results: Of 188 pts in the registry, 37 took PERT at enrollment/Visit 1. Mean (SD) age was 59.5 (10.1) years, and 20 pts were female (59%, n=34). Anxiety (38%) and depression (38%) were commonly ( >30%) reported medical histories; diabetes mellitus was reported in 19% of pts (Table 1). At least 41% of pts were taking another type of medication. The most frequent clinical manifestations at 6 months were diarrhea (29%) and bloating (29%). Most pts took a median of 6 PERT capsules/day and reported PERT greatly or somewhat improved their symptoms at visits ≤6 months (Figure 1A). Pts were generally satisfied with treatment convenience and effectiveness; global satisfaction appeared slightly increased over time (Figure 1B). Mean (SD) PCS-12 scores appeared stable at the first (40.93 [10.06], n=29) and 6-month (40.60 [9.77], n=20) visits and showed a positive trend on the MCS-12 (42.28 [11.03] and 45.91 [11.37], respectively).
Discussion: Most pts had comorbidities and a high pill burden, which could adversely affect treatment satisfaction and HRQoL. One limitation is that PERT use at baseline adherence/persistence could not be confirmed. Better understanding of how treatment satisfaction impacts treatment adherence/persistence and, subsequently, disease control or clinical outcomes may ultimately help improve HRQoL and reduce healthcare resource utilization in pts with EPI.
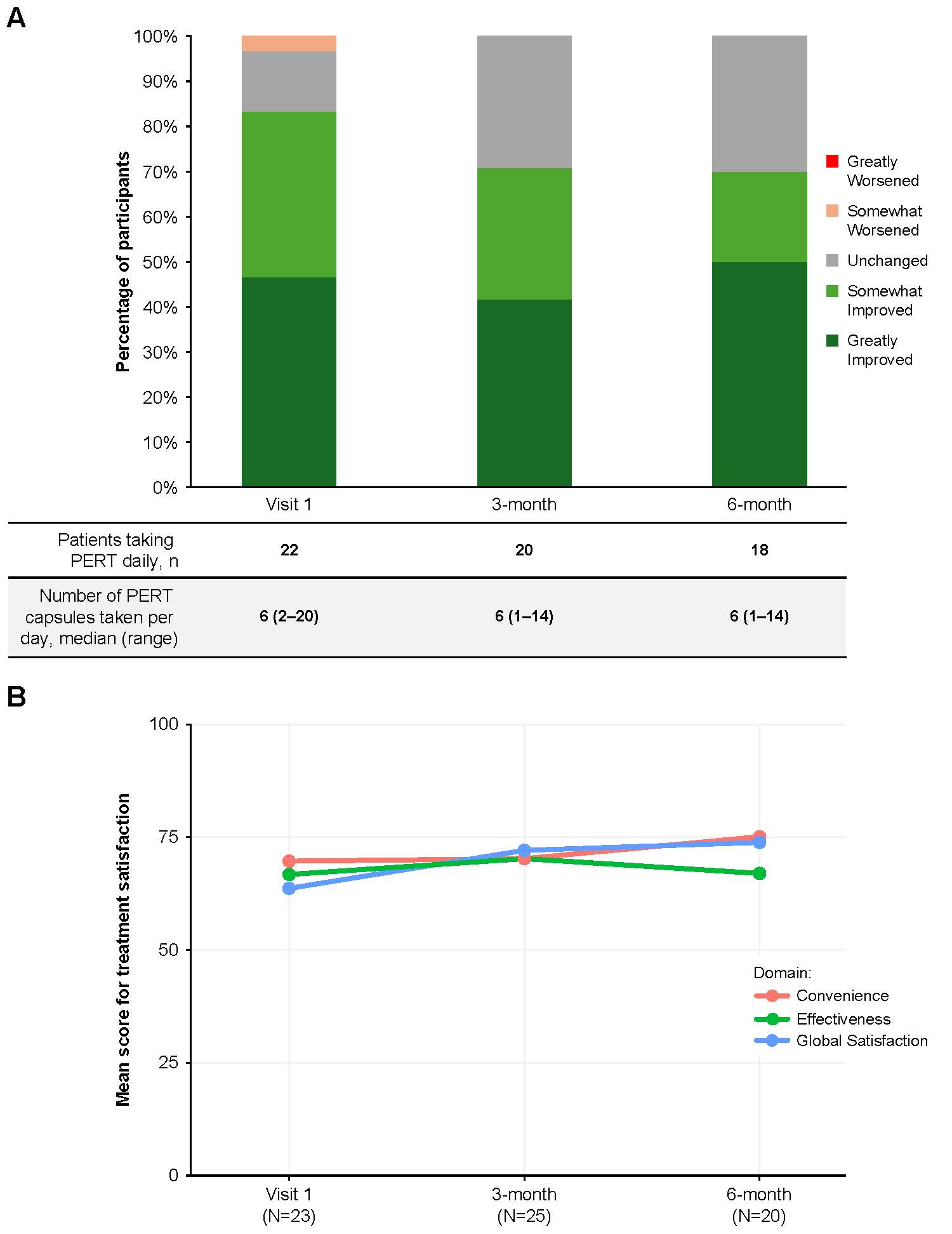
Figure: Figure 1A. Impact of PERT on symptoms over time up to 6 months as reported by patients taking PERT (pancrelipase; Zenpep® Delayed-Release Capsules). Figure 1B. Treatment satisfaction over time up to 6 months as reported by patients taking PERT (pancrelipase; Zenpep® Delayed-Release Capsules).
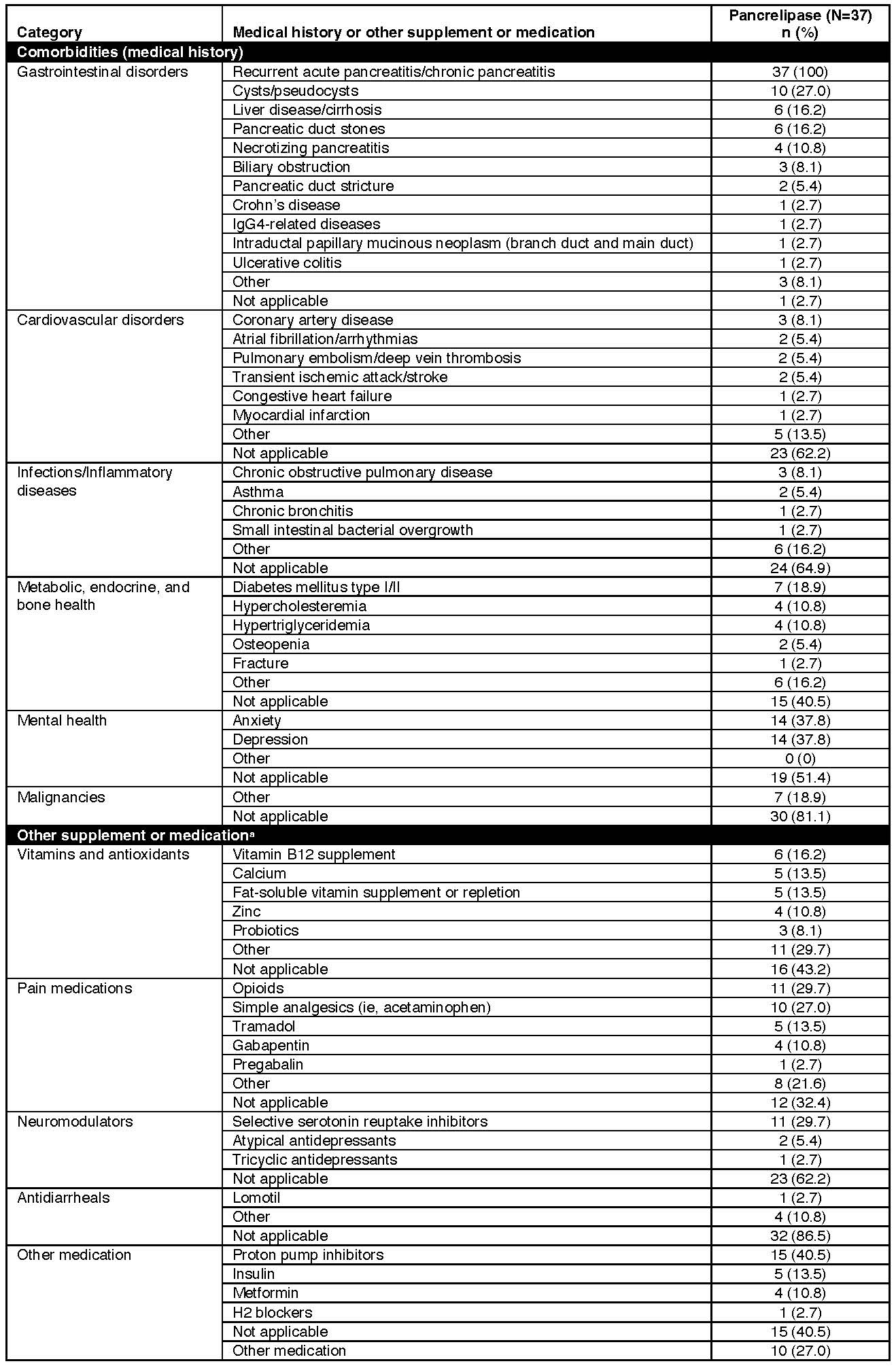
Figure: Comorbidities (medical history) and other supplement or medication at Visit 1
Disclosures:
Jodie Barkin: AbbVie – Advisor or Review Panel Member. Aimmune Therapeutics – Advisor or Review Panel Member. Amgen – Consultant. CorEvitas – Consultant. Iterative Health – Consultant. Medtronic – Consultant. MotusGI – Consultant.
Ismail Aouadi: Nestlé Health Science – Employee.
Jane Diamond indicated no relevant financial relationships.
Janine Twal: Nestlé Health Science – Employee.
Jennifer Pack: Nestlé Health Science – Employee.
Trudi Delk: Nestlé Health Science – Employee.
Cindy Frommor: Thermo Fisher Scientific – Employee.
Dianne Nguyen: Nestlé Health Science – Employee.
David C. Whitcomb: Ariel Precision Medicine – Consultant, Intellectual Property/Patents. Nestlé Health Science – Consultant. Orlando Health System – payment or honoraria. University of Arizona – payment or honoraria.
Jodie A. Barkin, MD, FACG1, Ismail Aouadi, MStat2, Jane Diamond, MS, FNP-BC3, Janine Twal, PharmD2, Jennifer Pack, MSN, ACNP-BC2, Trudi Delk, PharmD2, Cindy Frommor, BS4, Dianne Nguyen, MD2, David C. Whitcomb, MD, PhD5, 16, Characterizing Treatment Satisfaction and Health-Related Quality of Life of Patients With Exocrine Pancreatic Insufficiency (EPI) Taking Pancreatic Enzyme Replacement Therapy (PERT): Findings From the PACT-CP Registry, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Jodie A. Barkin, MD, FACG1, Ismail Aouadi, MStat2, Jane Diamond, MS, FNP-BC3, Janine Twal, PharmD2, Jennifer Pack, MSN, ACNP-BC2, Trudi Delk, PharmD2, Cindy Frommor, BS4, Dianne Nguyen, MD2, David C. Whitcomb, MD, PhD5
1University of Miami Miller School of Medicine, Miami, FL; 2Nestlé Health Science, Bridgewater, NJ; 3Gastroenterology Department, Mayo Clinic, Jacksonville, FL; 4PPD Observational Studies, Thermo Fisher Scientific, Waltham, MA; 5Department of Medicine, Cell Biology & Physiology and Human Genetics, Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh, UPMC and Ariel Precision Medicine, Pittsburgh, PA
Introduction: PERT is the mainstay of EPI treatment. Frequently, patients (pts) receiving PERT may have multiple comorbidities requiring polypharmacy, which can contribute to pill burden, influence treatment satisfaction, impact PERT adherence, and negatively affect health-related quality of life (HRQoL). The aim of this study was to assess these factors or aspects in pts with EPI with chronic pancreatitis (CP) or recurrent acute pancreatitis (RAP).
Methods: The US-based PAtient-CenTric Chronic Pancreatitis (PACT-CP) registry (NCT05762445) is a prospective, pt-driven registry designed to understand the unmet needs/burden of pts with EPI. Data were obtained from adults with suspected/confirmed EPI, a CP/RAP diagnosis, and taking PERT. Clinical characteristics, PERT use, treatment satisfaction (using the Treatment Satisfaction Questionnaire for Medication-9), and HRQoL (using physical [PCS-12] and mental [MCS-12] health scores) were collected from pts and/or their healthcare providers. Data from baseline to 6 months in pts receiving PERT (pancrelipase, Zenpep® Delayed-Release Capsules) are reported.
Results: Of 188 pts in the registry, 37 took PERT at enrollment/Visit 1. Mean (SD) age was 59.5 (10.1) years, and 20 pts were female (59%, n=34). Anxiety (38%) and depression (38%) were commonly ( >30%) reported medical histories; diabetes mellitus was reported in 19% of pts (Table 1). At least 41% of pts were taking another type of medication. The most frequent clinical manifestations at 6 months were diarrhea (29%) and bloating (29%). Most pts took a median of 6 PERT capsules/day and reported PERT greatly or somewhat improved their symptoms at visits ≤6 months (Figure 1A). Pts were generally satisfied with treatment convenience and effectiveness; global satisfaction appeared slightly increased over time (Figure 1B). Mean (SD) PCS-12 scores appeared stable at the first (40.93 [10.06], n=29) and 6-month (40.60 [9.77], n=20) visits and showed a positive trend on the MCS-12 (42.28 [11.03] and 45.91 [11.37], respectively).
Discussion: Most pts had comorbidities and a high pill burden, which could adversely affect treatment satisfaction and HRQoL. One limitation is that PERT use at baseline adherence/persistence could not be confirmed. Better understanding of how treatment satisfaction impacts treatment adherence/persistence and, subsequently, disease control or clinical outcomes may ultimately help improve HRQoL and reduce healthcare resource utilization in pts with EPI.

Figure: Figure 1A. Impact of PERT on symptoms over time up to 6 months as reported by patients taking PERT (pancrelipase; Zenpep® Delayed-Release Capsules). Figure 1B. Treatment satisfaction over time up to 6 months as reported by patients taking PERT (pancrelipase; Zenpep® Delayed-Release Capsules).

Figure: Comorbidities (medical history) and other supplement or medication at Visit 1
Disclosures:
Jodie Barkin: AbbVie – Advisor or Review Panel Member. Aimmune Therapeutics – Advisor or Review Panel Member. Amgen – Consultant. CorEvitas – Consultant. Iterative Health – Consultant. Medtronic – Consultant. MotusGI – Consultant.
Ismail Aouadi: Nestlé Health Science – Employee.
Jane Diamond indicated no relevant financial relationships.
Janine Twal: Nestlé Health Science – Employee.
Jennifer Pack: Nestlé Health Science – Employee.
Trudi Delk: Nestlé Health Science – Employee.
Cindy Frommor: Thermo Fisher Scientific – Employee.
Dianne Nguyen: Nestlé Health Science – Employee.
David C. Whitcomb: Ariel Precision Medicine – Consultant, Intellectual Property/Patents. Nestlé Health Science – Consultant. Orlando Health System – payment or honoraria. University of Arizona – payment or honoraria.
Jodie A. Barkin, MD, FACG1, Ismail Aouadi, MStat2, Jane Diamond, MS, FNP-BC3, Janine Twal, PharmD2, Jennifer Pack, MSN, ACNP-BC2, Trudi Delk, PharmD2, Cindy Frommor, BS4, Dianne Nguyen, MD2, David C. Whitcomb, MD, PhD5, 16, Characterizing Treatment Satisfaction and Health-Related Quality of Life of Patients With Exocrine Pancreatic Insufficiency (EPI) Taking Pancreatic Enzyme Replacement Therapy (PERT): Findings From the PACT-CP Registry, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


