Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3A: Liver / Functional
45 - A Placebo-Controlled Trial of CGRP Antagonist Rimegepant on Visceral Sensation and Symptoms in Participants With Non-Constipated IBS Pain (Late-Breaking Abstract)
Tuesday, October 28, 2025
3:35 PM - 3:45 PM PDT
Location: North Ballroom 120D

Ayah Matar, MD
Mayo Clinic
Rochester, MN
Late Breaking Abstract Presenter(s)
Ayah Matar, MD, Houssam Halawi, MD, Iris Wang, MD, Kara J. Jencks, MD, Irene Busciglio, BS, Deborah Eckert, BSN, RN, William S. Harmsen, MS, Michael Camilleri, MD, DSc, MACG
Mayo Clinic, Rochester, MN
Introduction: Calcitonin gene-related peptide (CGRP) is a neurotransmitter in digestive organs and is expressed by visceral afferents. CGRP significantly reduced visceral hypersensitivity to colonic distension in rats. Rimegepant, a CGRP receptor antagonist, is approved for episodic and preventive treatment of migraine pain. Our aim was to evaluate the safety and efficacy of rimegepant on abdominal pain (AP), bowel movements (BM), rectal compliance and sensation, gastrointestinal (GI) and colonic transit in participants with non-constipation irritable bowel syndrome (IBS) with AP.
Methods: We conducted a randomized, double-blind, placebo-controlled trial (NCT06221111) of oral rimegepant, 75mg every other day, which is the dose approved for preventative treatment of episodic migraine. Non-constipated IBS participants with pain were selected based on Rome III criteria. The trial included: 2-week run-in period, 4-week treatment phase, and 4-week post-treatment follow-up.
AP and BM were recorded in daily diaries during all 3 phases. Rectal compliance and sensory thresholds were assessed using a validated barostat method [stepwise distensions (0-44mmHg in 4mm increments)]. Sensory ratings of gas, urgency, and pain were obtained using 100mm visual analog scale (VAS) during phasic rectal distensions (RD) at 12, 24, and 36 mmHg applied in random order. GI and colonic transit were evaluated using standard scintigraphy. Statistical analyses were performed using ANCOVA, with sex, anxiety level, and baseline values of the respective parameters included as covariates.
Results: Demographics and effects on clinical and transit endpoints relative to baseline in the 2 groups are shown in Table 1. No differences were observed between groups in AP or BM at baseline or during treatment. Figure 1 shows rimegepant was associated with reductions in sensations of gas, urgency, pain during 24mmHg RD as well as gas and urgency sensations during 36mmHg RD (all P<u><0.05). Rimegepant increased rectal compliance (Table 1). No effects relative to baseline period were noted during washout period. No serious adverse events or ≥Grade 3 adverse events were reported.
Discussion: Rimegepant is associated with reduced rectal sensation and increased rectal compliance, both consistent with impact on visceral afferent function. This mechanistic study suggests that further clinical research of CGRP antagonists is warranted in patients with non-constipated IBS pain.
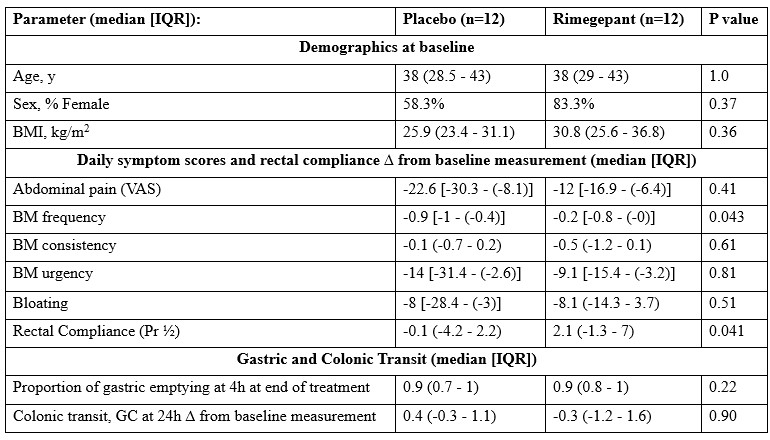
Figure: Table 1. Demographics at baseline and differences in primary and secondary endpoints between baseline and end of treatment for the two groups. P values based on ANCOVA with sex, anxiety, and baseline measurement of parameter as covariates.
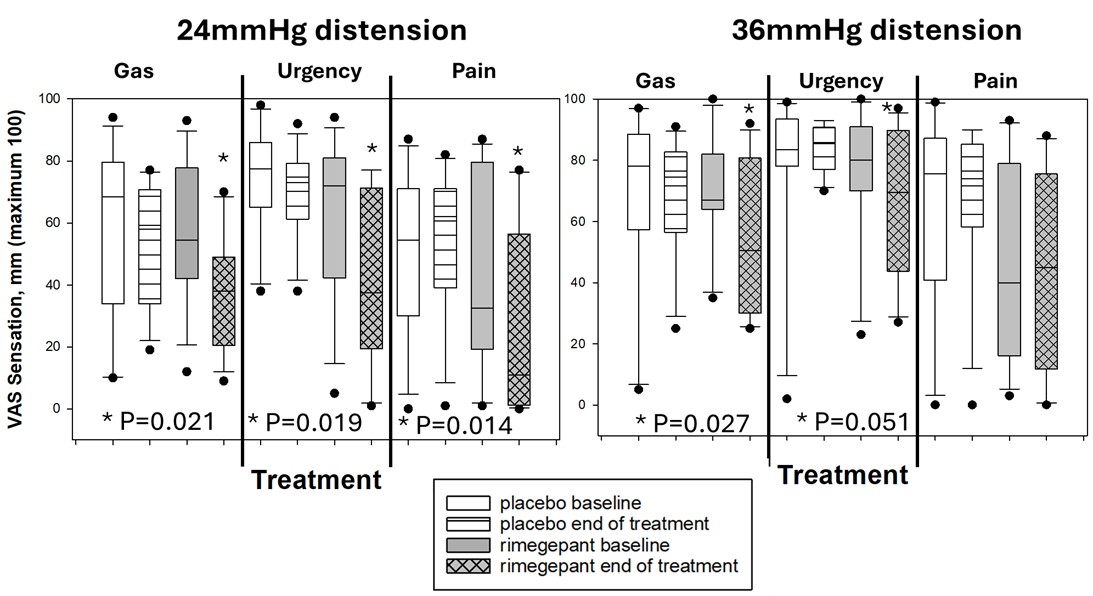
Figure: Figure 1. Boxplots showing significant differences in sensations of gas, urgency, and pain during 24mmHg and 36mmHg rectal distension between baseline and end of treatment for the two groups
Disclosures:
Ayah Matar indicated no relevant financial relationships.
Houssam Halawi indicated no relevant financial relationships.
Iris Wang indicated no relevant financial relationships.
Kara J. Jencks indicated no relevant financial relationships.
Irene Busciglio indicated no relevant financial relationships.
Deborah Eckert indicated no relevant financial relationships.
William S. Harmsen indicated no relevant financial relationships.
Michael Camilleri: Alfasigma – Consultant. Amylyx – Consultant. Biocodex – Grant/Research Support. BioKier – Consultant. Brightseed Bio – Consultant. Brightseed Bio – Grant/Research Support. Coloplast – Consultant. Dignify Therapeutics – Consultant, Stock Options. Intercept – Consultant. Invea – Consultant. Kallyope – Consultant. McDermott Will & Emery – Consultant. Medpace – Consultant. Monteresearch – Consultant. Neurogastrx – Consultant. NGM Biopharmaceuticals – Grant/Research Support. Pfizer – Grant/Research Support. Phenomix – Advisory Committee/Board Member, Stock Options. Renexxion – Consultant. SKYE Bioscience – Consultant. Sumitomoa – Consultant. Synlogic – Consultant. Vanda – Grant/Research Support.
Mayo Clinic, Rochester, MN
Introduction: Calcitonin gene-related peptide (CGRP) is a neurotransmitter in digestive organs and is expressed by visceral afferents. CGRP significantly reduced visceral hypersensitivity to colonic distension in rats. Rimegepant, a CGRP receptor antagonist, is approved for episodic and preventive treatment of migraine pain. Our aim was to evaluate the safety and efficacy of rimegepant on abdominal pain (AP), bowel movements (BM), rectal compliance and sensation, gastrointestinal (GI) and colonic transit in participants with non-constipation irritable bowel syndrome (IBS) with AP.
Methods: We conducted a randomized, double-blind, placebo-controlled trial (NCT06221111) of oral rimegepant, 75mg every other day, which is the dose approved for preventative treatment of episodic migraine. Non-constipated IBS participants with pain were selected based on Rome III criteria. The trial included: 2-week run-in period, 4-week treatment phase, and 4-week post-treatment follow-up.
AP and BM were recorded in daily diaries during all 3 phases. Rectal compliance and sensory thresholds were assessed using a validated barostat method [stepwise distensions (0-44mmHg in 4mm increments)]. Sensory ratings of gas, urgency, and pain were obtained using 100mm visual analog scale (VAS) during phasic rectal distensions (RD) at 12, 24, and 36 mmHg applied in random order. GI and colonic transit were evaluated using standard scintigraphy. Statistical analyses were performed using ANCOVA, with sex, anxiety level, and baseline values of the respective parameters included as covariates.
Results: Demographics and effects on clinical and transit endpoints relative to baseline in the 2 groups are shown in Table 1. No differences were observed between groups in AP or BM at baseline or during treatment. Figure 1 shows rimegepant was associated with reductions in sensations of gas, urgency, pain during 24mmHg RD as well as gas and urgency sensations during 36mmHg RD (all P<u><0.05). Rimegepant increased rectal compliance (Table 1). No effects relative to baseline period were noted during washout period. No serious adverse events or ≥Grade 3 adverse events were reported.
Discussion: Rimegepant is associated with reduced rectal sensation and increased rectal compliance, both consistent with impact on visceral afferent function. This mechanistic study suggests that further clinical research of CGRP antagonists is warranted in patients with non-constipated IBS pain.

Figure: Table 1. Demographics at baseline and differences in primary and secondary endpoints between baseline and end of treatment for the two groups. P values based on ANCOVA with sex, anxiety, and baseline measurement of parameter as covariates.

Figure: Figure 1. Boxplots showing significant differences in sensations of gas, urgency, and pain during 24mmHg and 36mmHg rectal distension between baseline and end of treatment for the two groups
Disclosures:
Ayah Matar indicated no relevant financial relationships.
Houssam Halawi indicated no relevant financial relationships.
Iris Wang indicated no relevant financial relationships.
Kara J. Jencks indicated no relevant financial relationships.
Irene Busciglio indicated no relevant financial relationships.
Deborah Eckert indicated no relevant financial relationships.
William S. Harmsen indicated no relevant financial relationships.
Michael Camilleri: Alfasigma – Consultant. Amylyx – Consultant. Biocodex – Grant/Research Support. BioKier – Consultant. Brightseed Bio – Consultant. Brightseed Bio – Grant/Research Support. Coloplast – Consultant. Dignify Therapeutics – Consultant, Stock Options. Intercept – Consultant. Invea – Consultant. Kallyope – Consultant. McDermott Will & Emery – Consultant. Medpace – Consultant. Monteresearch – Consultant. Neurogastrx – Consultant. NGM Biopharmaceuticals – Grant/Research Support. Pfizer – Grant/Research Support. Phenomix – Advisory Committee/Board Member, Stock Options. Renexxion – Consultant. SKYE Bioscience – Consultant. Sumitomoa – Consultant. Synlogic – Consultant. Vanda – Grant/Research Support.


