Monday Poster Session
Category: IBD
P3235 - Comparative Study of Efficacy and Safety of Infliximab vs Risankizumab in Moderate to Severe Crohn's Disease: A Systematic Review and Network Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Gillan S. Quirequire, MD, RN (she/her/hers)
NYC Health +/ Woodhull
Brooklyn, NY
Presenting Author(s)
Gillan S. Quirequire, MD, RN1, Manuel Martinez, MD, FACG2
1NYC Health +/ Woodhull, Brooklyn, NY; 2NYC Health + Hospitals/Woodhull, Brooklyn, NY
Introduction: Bayesian network meta-analysis was utilized to compare the efficacy and safety of Infliximab and Risankizumab for treatment of moderate-severe Crohn Disease (CD). Noticeably, Risankizumab was superior in endoscopic remission rate and was non-inferior in clinical remission rate both in induction and maintenance phase.
Methods: Systematic literature search via PubMed. Criteria were the following:1-randomized clinical trials 2-Population adult patients with moderate to severe Crohn Disease 3-Interventions Risankizumab induction dosage is 600 mg and maintenance dosage of 180 mg or 360 mg vs Infliximab 5 mg or 10 mg 4-Outcomes Endoscopic Remission SES- CD < 4, Clinical Remission CDAI < 150; incidence of any adverse events. The meta-analysis used OpenBUGS or JAGS application for Bayesian analysis, with R being used for data synthesis and visualization. Cochrane risk bias was categorized as Low, High or Unclear.
Results: A total of 15 randomized control studies recruited 4579 patients during different periods and various treatment regimens-11 Infliximab trials and 6 Risankizumab trials. Infliximab’s endoscopic induction remission rate of 90.6% was below 97.5% threshold of being significant OR 2.284 95% CrI: 0.457-12.629; while Risankizumab was 99.6% OR 3.452 95% CrI: 1.402-9.088. The between-study heterogeneity I² 15% indicated low to moderate variability in treatment effects across all studies. Risankizumab 72.8% had best treatment Rank1 and Infliximab 62.3% Rank2. Infliximab’s endoscopy remission rate 95.4% during maintenance while Risankizumab was 95.8% with an I²10%. Infliximab 51.6% and Risankizumab 47.4% had nearly equal outcomes Rank 1. Clinical remission rates during induction of Infliximab 96.2% and Risankizumab 96.6% were not significantly different, but both Infliximab 51.6% and Risankizumab 47.4% were the most effective. Clinical remission rate during maintenance of Infliximab 95.8% compared to Risankizumab 81.6% were not significant, I² 12%. Infliximab adverse events reported 4 lymphoma and 1 tuberculosis.
Discussion: Infliximab had more adverse effects with a yearly cost of $50,510 compared with Risankizumab $41,338 for first year and $29,855/year. Patients using Infliximab warrant regular infusion center visits, while Risankizumab 600 mg IV infusion for induction followed by maintenance therapy 360 mg subcutaneously self-administered at week 12, and every 8 weeks thereafter making it a cost-effective alternative.
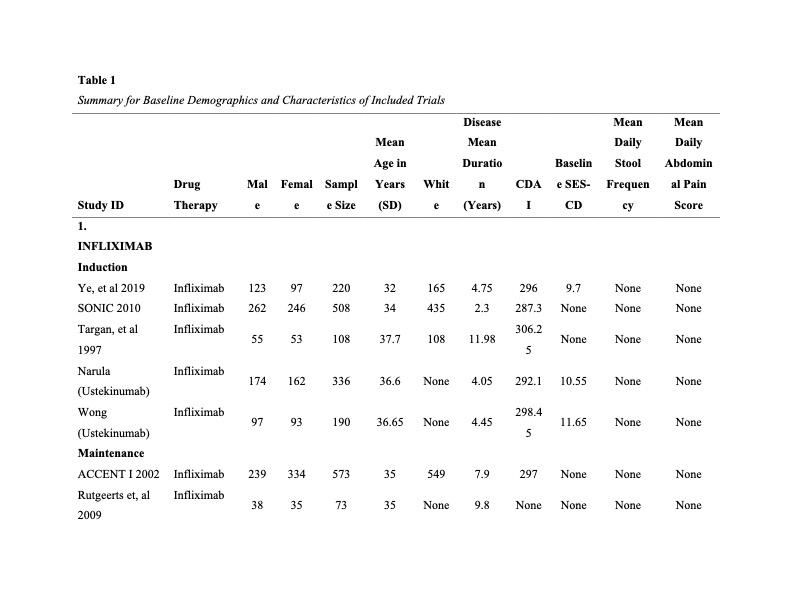
Figure: Summary for Baseline Demographics and Characteristics of Included Trials page 1
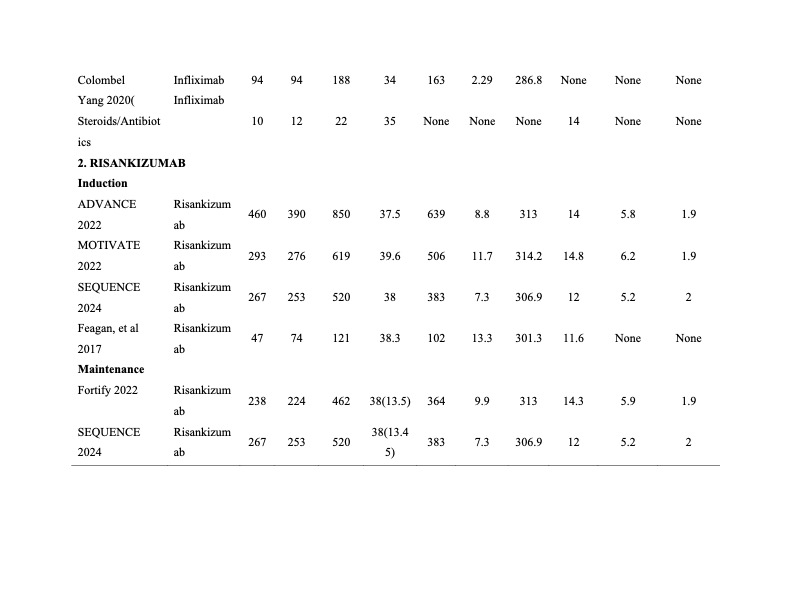
Figure: Summary for Baseline Demographics and Characteristics of Included Trials page 2
Disclosures:
Gillan Quirequire indicated no relevant financial relationships.
Manuel Martinez indicated no relevant financial relationships.
Gillan S. Quirequire, MD, RN1, Manuel Martinez, MD, FACG2. P3235 - Comparative Study of Efficacy and Safety of Infliximab vs Risankizumab in Moderate to Severe Crohn's Disease: A Systematic Review and Network Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1NYC Health +/ Woodhull, Brooklyn, NY; 2NYC Health + Hospitals/Woodhull, Brooklyn, NY
Introduction: Bayesian network meta-analysis was utilized to compare the efficacy and safety of Infliximab and Risankizumab for treatment of moderate-severe Crohn Disease (CD). Noticeably, Risankizumab was superior in endoscopic remission rate and was non-inferior in clinical remission rate both in induction and maintenance phase.
Methods: Systematic literature search via PubMed. Criteria were the following:1-randomized clinical trials 2-Population adult patients with moderate to severe Crohn Disease 3-Interventions Risankizumab induction dosage is 600 mg and maintenance dosage of 180 mg or 360 mg vs Infliximab 5 mg or 10 mg 4-Outcomes Endoscopic Remission SES- CD < 4, Clinical Remission CDAI < 150; incidence of any adverse events. The meta-analysis used OpenBUGS or JAGS application for Bayesian analysis, with R being used for data synthesis and visualization. Cochrane risk bias was categorized as Low, High or Unclear.
Results: A total of 15 randomized control studies recruited 4579 patients during different periods and various treatment regimens-11 Infliximab trials and 6 Risankizumab trials. Infliximab’s endoscopic induction remission rate of 90.6% was below 97.5% threshold of being significant OR 2.284 95% CrI: 0.457-12.629; while Risankizumab was 99.6% OR 3.452 95% CrI: 1.402-9.088. The between-study heterogeneity I² 15% indicated low to moderate variability in treatment effects across all studies. Risankizumab 72.8% had best treatment Rank1 and Infliximab 62.3% Rank2. Infliximab’s endoscopy remission rate 95.4% during maintenance while Risankizumab was 95.8% with an I²10%. Infliximab 51.6% and Risankizumab 47.4% had nearly equal outcomes Rank 1. Clinical remission rates during induction of Infliximab 96.2% and Risankizumab 96.6% were not significantly different, but both Infliximab 51.6% and Risankizumab 47.4% were the most effective. Clinical remission rate during maintenance of Infliximab 95.8% compared to Risankizumab 81.6% were not significant, I² 12%. Infliximab adverse events reported 4 lymphoma and 1 tuberculosis.
Discussion: Infliximab had more adverse effects with a yearly cost of $50,510 compared with Risankizumab $41,338 for first year and $29,855/year. Patients using Infliximab warrant regular infusion center visits, while Risankizumab 600 mg IV infusion for induction followed by maintenance therapy 360 mg subcutaneously self-administered at week 12, and every 8 weeks thereafter making it a cost-effective alternative.

Figure: Summary for Baseline Demographics and Characteristics of Included Trials page 1

Figure: Summary for Baseline Demographics and Characteristics of Included Trials page 2
Disclosures:
Gillan Quirequire indicated no relevant financial relationships.
Manuel Martinez indicated no relevant financial relationships.
Gillan S. Quirequire, MD, RN1, Manuel Martinez, MD, FACG2. P3235 - Comparative Study of Efficacy and Safety of Infliximab vs Risankizumab in Moderate to Severe Crohn's Disease: A Systematic Review and Network Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
