Monday Poster Session
Category: Interventional Endoscopy
P3514 - The Effectiveness of Forceps-Assisted Cannulation for Difficult Cannulation During ERCP: Results of the SOCCER Randomized Controlled Trial
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Steven M. Hadley, Jr., BA
Feinberg School of Medicine, Northwestern University
Chicago, IL
Presenting Author(s)
Steven M. Hadley, BA1, Jessica I. Chevalier, BS2, Grace E. Tomasetti, BS2, Julia C. Hill, BA2, Madison C. Duclos, MPH2, David A. Klibansky, MD2, Heiko Pohl, MD, MS2, Corey A. Siegel, MD, MS3, Arifa Toor, MD2, Steven P. Bensen, MD2, Jeffrey M. Adler, MD2, Stuart R. Gordon, MD2, Timothy B. Gardner, MD, MS2
1Feinberg School of Medicine, Northwestern University, Chicago, IL; 2Dartmouth Hitchcock Medical Center, Lebanon, NH; 3Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, NH
Introduction: Forceps-assisted cannulation has been reported to facilitate difficult papillary cannulation during ERCP, especially in the context of abnormal papillary anatomy or associated papillary diverticula. We performed a randomized, controlled trial to evaluate if forceps-assisted cannulation improves cannulation success rates, reduces the incidence of difficult cannulations, and decreases the risk of post-ERCP pancreatitis (PEP).
Methods: 152 patients with difficult papillary cannulation during ERCP were randomized to cannulation with or without forceps. Difficult cannulation was defined as: papilla in/on the rim of a diverticulum, difficult cannulation (defined as 5 or more attempts, 5 or more minutes, or 2 or more unintended PD wire passages), redundant tissue overlaying the papilla, or a type 2, 3, or 4 papilla. The primary clinical outcome was rate of successful cannulation.
Results: 70 patients underwent forceps-assisted cannulation and 81 did not use forceps. Forceps patients were younger (62 vs. 68 years p=0.009), but otherwise baseline demographics, ERCP indication, trainee involvement and papilla classification were similar with failed initial cannulation the most common reason for enrollment. 100% of patients in the forceps-assisted group vs. 83.9% in the no forceps group (p< 0.001) underwent successful cannulation. All 13 patients in the no forceps group who crossed over to the forceps group had successful cannulation. While not statistically significant, the difficult cannulation rate (57.1 vs. 69.1, p=0.132) was higher in the no forceps group and the PEP rate was low in both groups (5.7 vs 3.7, p=0.705). The no forceps group had significantly more cannulation attempts following randomization compared to forceps (14.0 vs. 8.3, p=0.026). Forceps-assisted resulted in decreased cannulation time after randomization than no forceps, though this value failed to reach significance (9.1 vs. 13.1 minutes, p=0.081). Most subjects had type 1 papillae (72.2%, 109/151). The incidence of the other papillae was: 7.3% (11/151) type 2, 14.5% (22/151) type 3, and 6.0% (9/151) type 4. Forceps had significantly higher cannulation rates among type 1 compared to no forceps (100% vs. 84%, p=0.005). Forceps also had higher success rates in type 2 (100% vs. 60%, p=0.182) and type 3 (100% vs. 88%, p=0.364), though these values did not reach significance.
Discussion: Using forceps-assisted technique to overcome difficult cannulation improves ERCP cannulation success rates.
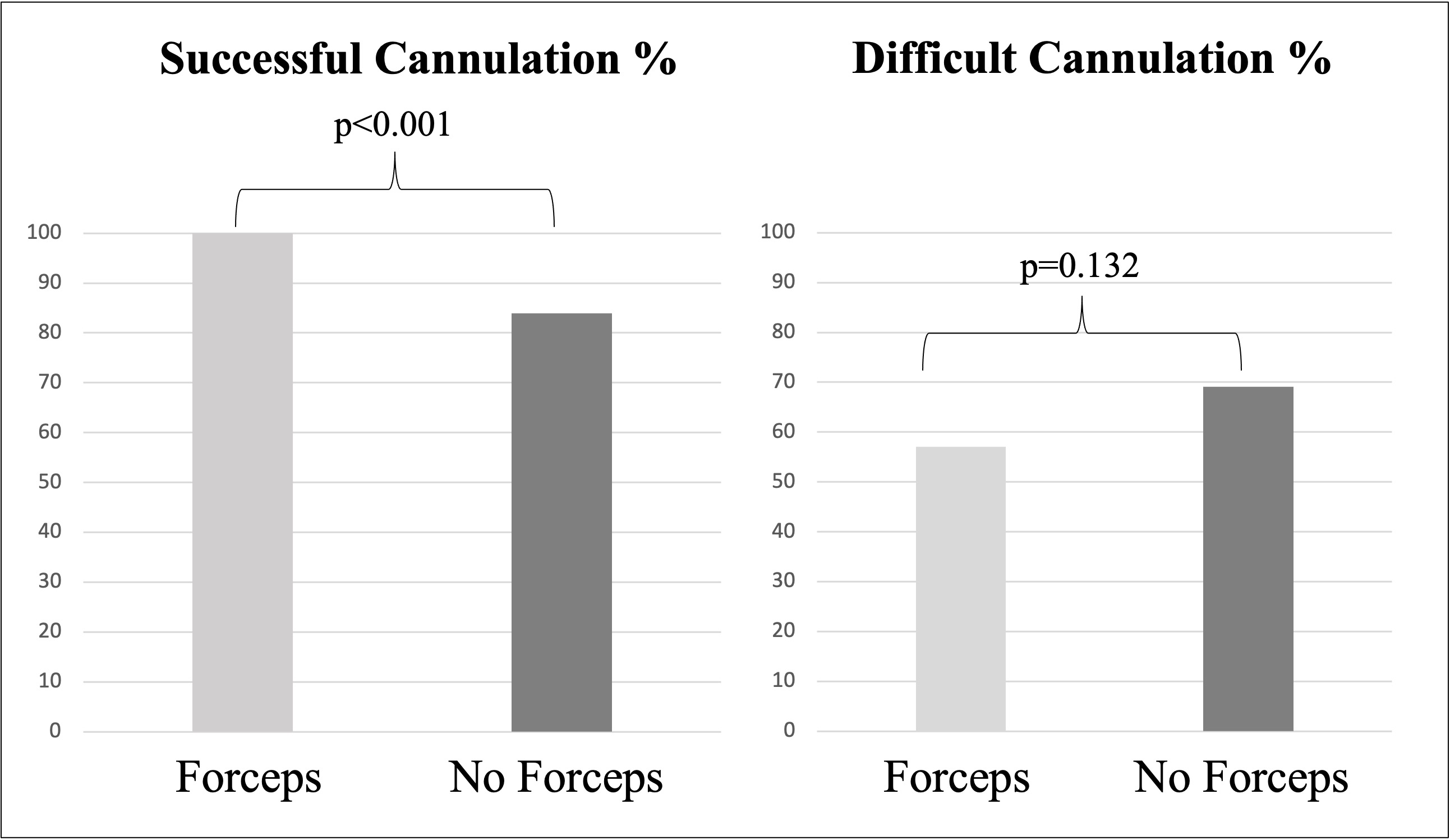
Figure: Figure 1: Primary clinical outcomes
Disclosures:
Steven Hadley indicated no relevant financial relationships.
Jessica Chevalier indicated no relevant financial relationships.
Grace Tomasetti indicated no relevant financial relationships.
Julia Hill indicated no relevant financial relationships.
Madison Duclos indicated no relevant financial relationships.
David Klibansky indicated no relevant financial relationships.
Heiko Pohl indicated no relevant financial relationships.
Corey A. Siegel: AbbVie – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Boomerang – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Johnson and Johnson – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Lilly – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Napo Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Path Healthcare – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Prometheus Labs – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Trellus Health – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau.
Arifa Toor indicated no relevant financial relationships.
Steven Bensen indicated no relevant financial relationships.
Jeffrey Adler indicated no relevant financial relationships.
Stuart Gordon: Boston Scientific – Consultant.
Timothy Gardner indicated no relevant financial relationships.
Steven M. Hadley, BA1, Jessica I. Chevalier, BS2, Grace E. Tomasetti, BS2, Julia C. Hill, BA2, Madison C. Duclos, MPH2, David A. Klibansky, MD2, Heiko Pohl, MD, MS2, Corey A. Siegel, MD, MS3, Arifa Toor, MD2, Steven P. Bensen, MD2, Jeffrey M. Adler, MD2, Stuart R. Gordon, MD2, Timothy B. Gardner, MD, MS2. P3514 - The Effectiveness of Forceps-Assisted Cannulation for Difficult Cannulation During ERCP: Results of the SOCCER Randomized Controlled Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Feinberg School of Medicine, Northwestern University, Chicago, IL; 2Dartmouth Hitchcock Medical Center, Lebanon, NH; 3Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, NH
Introduction: Forceps-assisted cannulation has been reported to facilitate difficult papillary cannulation during ERCP, especially in the context of abnormal papillary anatomy or associated papillary diverticula. We performed a randomized, controlled trial to evaluate if forceps-assisted cannulation improves cannulation success rates, reduces the incidence of difficult cannulations, and decreases the risk of post-ERCP pancreatitis (PEP).
Methods: 152 patients with difficult papillary cannulation during ERCP were randomized to cannulation with or without forceps. Difficult cannulation was defined as: papilla in/on the rim of a diverticulum, difficult cannulation (defined as 5 or more attempts, 5 or more minutes, or 2 or more unintended PD wire passages), redundant tissue overlaying the papilla, or a type 2, 3, or 4 papilla. The primary clinical outcome was rate of successful cannulation.
Results: 70 patients underwent forceps-assisted cannulation and 81 did not use forceps. Forceps patients were younger (62 vs. 68 years p=0.009), but otherwise baseline demographics, ERCP indication, trainee involvement and papilla classification were similar with failed initial cannulation the most common reason for enrollment. 100% of patients in the forceps-assisted group vs. 83.9% in the no forceps group (p< 0.001) underwent successful cannulation. All 13 patients in the no forceps group who crossed over to the forceps group had successful cannulation. While not statistically significant, the difficult cannulation rate (57.1 vs. 69.1, p=0.132) was higher in the no forceps group and the PEP rate was low in both groups (5.7 vs 3.7, p=0.705). The no forceps group had significantly more cannulation attempts following randomization compared to forceps (14.0 vs. 8.3, p=0.026). Forceps-assisted resulted in decreased cannulation time after randomization than no forceps, though this value failed to reach significance (9.1 vs. 13.1 minutes, p=0.081). Most subjects had type 1 papillae (72.2%, 109/151). The incidence of the other papillae was: 7.3% (11/151) type 2, 14.5% (22/151) type 3, and 6.0% (9/151) type 4. Forceps had significantly higher cannulation rates among type 1 compared to no forceps (100% vs. 84%, p=0.005). Forceps also had higher success rates in type 2 (100% vs. 60%, p=0.182) and type 3 (100% vs. 88%, p=0.364), though these values did not reach significance.
Discussion: Using forceps-assisted technique to overcome difficult cannulation improves ERCP cannulation success rates.

Figure: Figure 1: Primary clinical outcomes
Disclosures:
Steven Hadley indicated no relevant financial relationships.
Jessica Chevalier indicated no relevant financial relationships.
Grace Tomasetti indicated no relevant financial relationships.
Julia Hill indicated no relevant financial relationships.
Madison Duclos indicated no relevant financial relationships.
David Klibansky indicated no relevant financial relationships.
Heiko Pohl indicated no relevant financial relationships.
Corey A. Siegel: AbbVie – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Boomerang – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Johnson and Johnson – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Lilly – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Napo Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Path Healthcare – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Prometheus Labs – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Trellus Health – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau.
Arifa Toor indicated no relevant financial relationships.
Steven Bensen indicated no relevant financial relationships.
Jeffrey Adler indicated no relevant financial relationships.
Stuart Gordon: Boston Scientific – Consultant.
Timothy Gardner indicated no relevant financial relationships.
Steven M. Hadley, BA1, Jessica I. Chevalier, BS2, Grace E. Tomasetti, BS2, Julia C. Hill, BA2, Madison C. Duclos, MPH2, David A. Klibansky, MD2, Heiko Pohl, MD, MS2, Corey A. Siegel, MD, MS3, Arifa Toor, MD2, Steven P. Bensen, MD2, Jeffrey M. Adler, MD2, Stuart R. Gordon, MD2, Timothy B. Gardner, MD, MS2. P3514 - The Effectiveness of Forceps-Assisted Cannulation for Difficult Cannulation During ERCP: Results of the SOCCER Randomized Controlled Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

