Sunday Poster Session
Category: Esophagus
P0610 - Using Health Insurance Claims Data to Determine the Severity of Eosinophilic Esophagitis in Patients in the USA: A Retrospective Cohort Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Eric D. Shah, MD, MBA
Division of Gastroenterology and Hepatology, University of Michigan
Ann Arbor, MI
Presenting Author(s)
Eric D. Shah, MD, MBA1, Bridgett Goodwin, PhD2, Yiyan Liu, PhD2, Bertha de Los Santos, PharmD3, Siddhi Korgaonkar, PhD4, Juliana Meyers, MA4, Carolyn R. Schaeffer-Koziol, PhD2, Brian Terreri, PharmD2, Evan S. Dellon, MD, MPH5
1Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, MI; 2Takeda Pharmaceuticals USA, Inc., Lexington, MA; 3Takeda Pharmaceuticals USA, Inc., Chicago, IL; 4RTI Health Solutions, Research Triangle Park, NC; 5Center for Esophageal Diseases and Swallowing, University of North Carolina School of Medicine, Chapel Hill, NC
Introduction: Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease. If untreated/inadequately treated, the disease can progress from an inflammatory to a more severe, fibrostenotic phenotype. There is increasing interest in understanding the effect of disease severity on the burden of EoE.
Methods: An algorithm was developed, based on the Index of Severity for EoE (I-SEE), to classify EoE disease severity using EoE-related diagnoses, procedures and complications identifiable in US health insurance claims data. This analysis obtained data from a retrospective, observational cohort study using the Merative MarketScan Commercial, Medicare Supplemental and Medicaid databases over 3 years (July 1, 2020–June 30, 2023). Patients were included if they had ≥1 inpatient/outpatient claim with a diagnosis code for EoE (ICD-10-CM: K20.0; the date of the first claim during the selection window [July 1, 2021–June 30, 2022] was the index date) and 12 months of continuous health plan enrollment before and after the index date (baseline and follow-up periods, respectively). Patients were excluded if they had a diagnosis of eosinophilic gastritis/eosinophilic gastroenteritis (ICD-10-CM: K52.81) during the study. Using the algorithm developed (Table 1), patients were classified as having mild, moderate or severe EoE based on the EoE-related diagnoses, procedures and complications identifiable in the baseline period + 30 days after the index date. Patient demographics and clinical characteristics were reported, stratified by EoE severity.
Results: Overall, 19,169 patients with EoE were identified. The mean (SD) age of patients was 35.8 (18.5) years. The majority of patients were male (60.3%) and most had commercial insurance (73.8%). EoE disease severity was classified as mild, moderate and severe in 67.5%, 24.9% and 7.6% of patients, respectively. Compared with patients with mild or moderate EoE, those with severe disease were younger and a higher proportion were male. Patients with severe EoE also had lower levels of commercial insurance coverage, and the majority had an insurance claim for EoE during the baseline period (i.e. established EoE) (Table 2).
Discussion: This algorithm provides a useful tool for determining EoE disease severity outside of clinical practice using health insurance claims. The proportions of patients with mild, moderate and severe EoE were similar to those reported in clinical studies using the I-SEE, supporting the validity of this method.
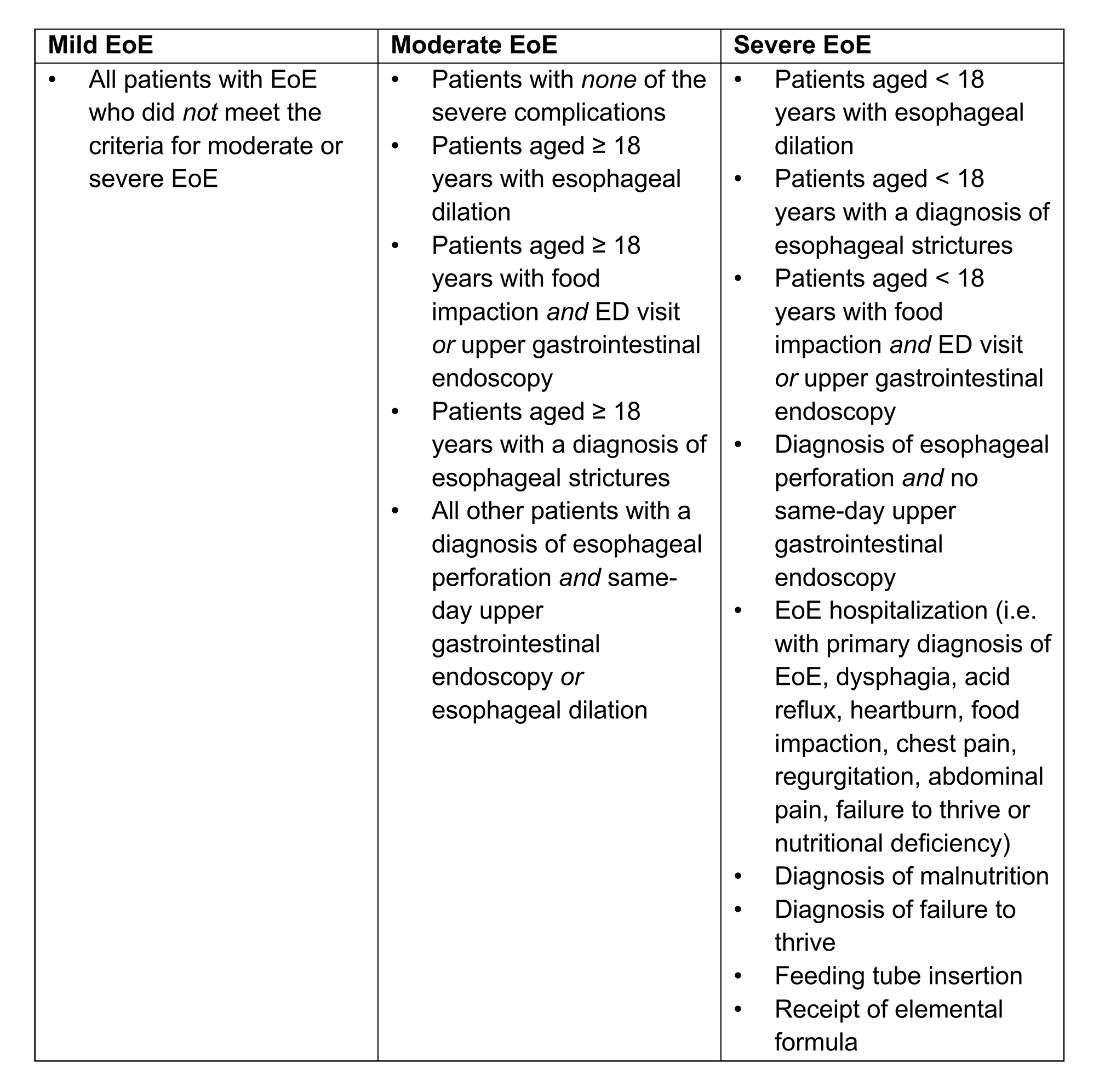
Figure: Table 1. EoE severity scoring algorithm using EoE-related diagnoses, procedures and complications identifiable in the health insurance claims data.
EoE-related diagnoses, procedures and complications were identified over the 12-month baseline period + 30 days after the index date.
EoE, eosinophilic esophagitis; ED, emergency department.
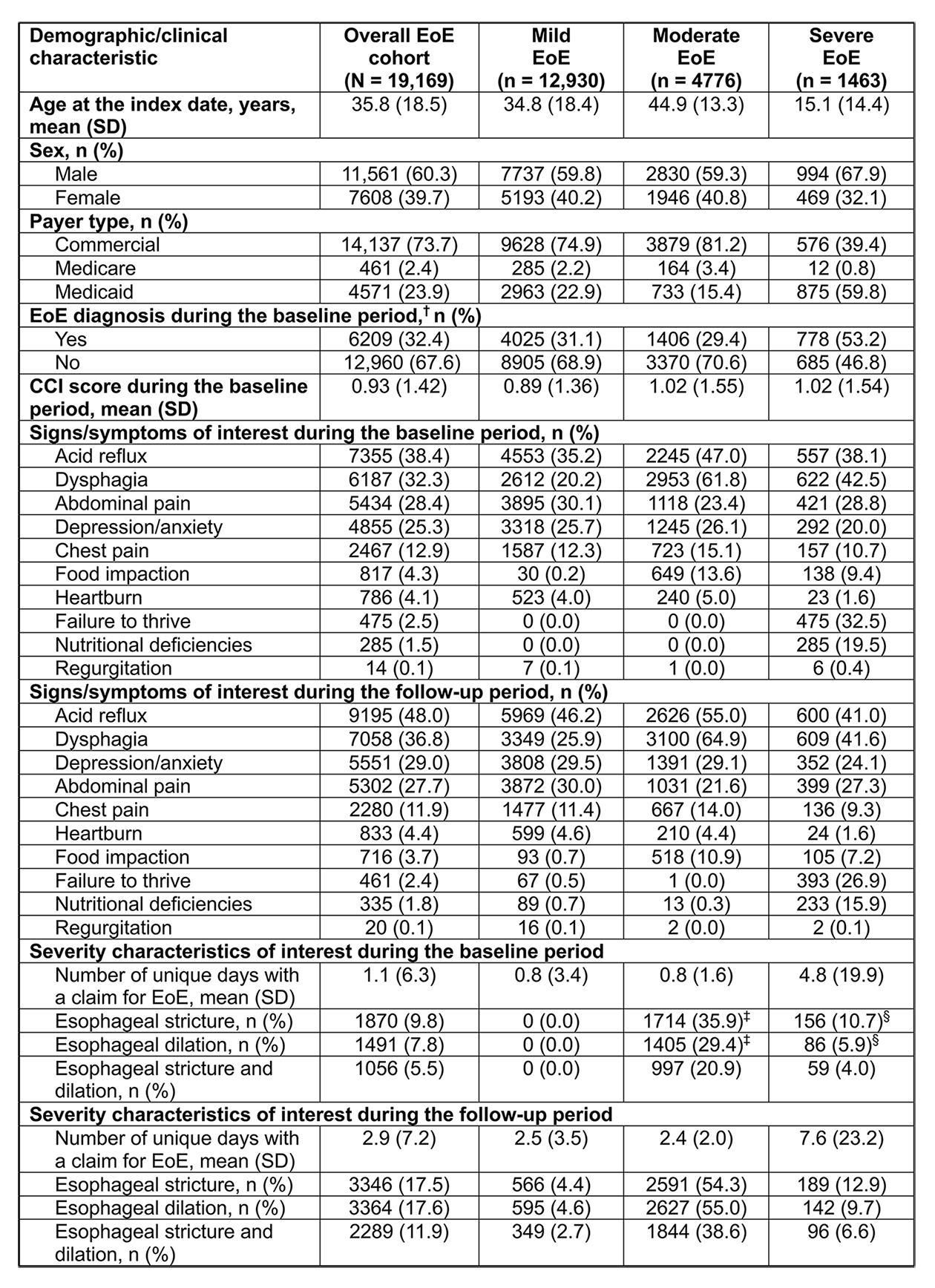
Figure: Table 2. Demographics and clinical characteristics of patients stratified by EoE disease severity during the baseline period + 30 days after the index date.*
*Severity was classified based on EoE-related diagnoses, procedures and complications identified over the 12-month baseline period + 30 days after the index date.
†Patients with the answer ‘Yes’ to this question likely had established EoE (i.e. before the index date), whereas those with the answer ‘No’ were likely to be incident cases. Diagnosis was defined as a patient having ≥1 claim containing the diagnosis code for EoE (ICD-10-CM: K20.0).
‡For the purposes of the algorithm, patients qualifying with these procedures/diagnoses had to be aged ≥ 18 years.
§For the purposes of the algorithm, patients qualifying with these procedures/diagnoses had to be aged < 18 years.
CCI, Charlson Comorbidity Index; EoE, eosinophilic esophagitis; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; SD, standard deviation.
Disclosures:
Eric Shah: Ardelyx – Consultant. Mahana Therapeutics – Consultant. NeurAxis – Consultant. Salix Pharmaceuticals – Consultant. Takeda Pharmaceuticals – Consultant. University of Michigan – Intellectual Property/Patents.
Bridgett Goodwin: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Yiyan Liu: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Bertha de Los Santos: Takeda Pharmaceuticals USA, Inc. – Employee.
Siddhi Korgaonkar: RTI Health Solutions – Employee.
Juliana Meyers: RTI Health Solutions – Employee.
Carolyn Schaeffer-Koziol: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Brian Terreri: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Evan Dellon: AbbVie – Consultant. Adare/Ellodi – Consultant, Grant/Research Support. Akesobio – Consultant. Alfasigma – Consultant. ALK – Consultant. Allakos – Consultant, Grant/Research Support. Amgen – Consultant. Apogee – Consultant. Apollo – Consultant. Aqilion – Consultant, Grant/Research Support. Arena/Pfizer – Consultant, Grant/Research Support. Aslan – Consultant. AstraZeneca – Consultant, Grant/Research Support. Avir – Consultant. Biocryst – Consultant. Bryn – Consultant. Calypso – Consultant. Celgene/Receptos/Bristol Myers Squibb – Consultant, Grant/Research Support. Celldex – Consultant, Grant/Research Support. Dr. Falk Pharma – Consultant. EsoCap – Consultant. Eupraxia – Consultant, Grant/Research Support. Ferring – Consultant, Grant/Research Support. GI Reviewers – Consultant. GSK – Consultant, Grant/Research Support. Holoclara – Consultant, Grant/Research Support. Invea – Consultant, Grant/Research Support. Knightpoint – Consultant. LucidDx – Consultant. Meritage – Grant/Research Support. Miraca – Grant/Research Support. Morphic – Consultant. Nexstone Immunology/Uniquity – Consultant. Nutricia – Consultant, Grant/Research Support. Parexel/Calyx – Consultant. Phathom – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Revolo – Consultant, Grant/Research Support. Robarts/Alimentiv – Consultant. Sanofi – Consultant, Grant/Research Support. Shire/Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Third Harmonic Bio – Consultant. Uniquity – Grant/Research Support. Upstream Bio – Consultant.
Eric D. Shah, MD, MBA1, Bridgett Goodwin, PhD2, Yiyan Liu, PhD2, Bertha de Los Santos, PharmD3, Siddhi Korgaonkar, PhD4, Juliana Meyers, MA4, Carolyn R. Schaeffer-Koziol, PhD2, Brian Terreri, PharmD2, Evan S. Dellon, MD, MPH5. P0610 - Using Health Insurance Claims Data to Determine the Severity of Eosinophilic Esophagitis in Patients in the USA: A Retrospective Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, MI; 2Takeda Pharmaceuticals USA, Inc., Lexington, MA; 3Takeda Pharmaceuticals USA, Inc., Chicago, IL; 4RTI Health Solutions, Research Triangle Park, NC; 5Center for Esophageal Diseases and Swallowing, University of North Carolina School of Medicine, Chapel Hill, NC
Introduction: Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease. If untreated/inadequately treated, the disease can progress from an inflammatory to a more severe, fibrostenotic phenotype. There is increasing interest in understanding the effect of disease severity on the burden of EoE.
Methods: An algorithm was developed, based on the Index of Severity for EoE (I-SEE), to classify EoE disease severity using EoE-related diagnoses, procedures and complications identifiable in US health insurance claims data. This analysis obtained data from a retrospective, observational cohort study using the Merative MarketScan Commercial, Medicare Supplemental and Medicaid databases over 3 years (July 1, 2020–June 30, 2023). Patients were included if they had ≥1 inpatient/outpatient claim with a diagnosis code for EoE (ICD-10-CM: K20.0; the date of the first claim during the selection window [July 1, 2021–June 30, 2022] was the index date) and 12 months of continuous health plan enrollment before and after the index date (baseline and follow-up periods, respectively). Patients were excluded if they had a diagnosis of eosinophilic gastritis/eosinophilic gastroenteritis (ICD-10-CM: K52.81) during the study. Using the algorithm developed (Table 1), patients were classified as having mild, moderate or severe EoE based on the EoE-related diagnoses, procedures and complications identifiable in the baseline period + 30 days after the index date. Patient demographics and clinical characteristics were reported, stratified by EoE severity.
Results: Overall, 19,169 patients with EoE were identified. The mean (SD) age of patients was 35.8 (18.5) years. The majority of patients were male (60.3%) and most had commercial insurance (73.8%). EoE disease severity was classified as mild, moderate and severe in 67.5%, 24.9% and 7.6% of patients, respectively. Compared with patients with mild or moderate EoE, those with severe disease were younger and a higher proportion were male. Patients with severe EoE also had lower levels of commercial insurance coverage, and the majority had an insurance claim for EoE during the baseline period (i.e. established EoE) (Table 2).
Discussion: This algorithm provides a useful tool for determining EoE disease severity outside of clinical practice using health insurance claims. The proportions of patients with mild, moderate and severe EoE were similar to those reported in clinical studies using the I-SEE, supporting the validity of this method.

Figure: Table 1. EoE severity scoring algorithm using EoE-related diagnoses, procedures and complications identifiable in the health insurance claims data.
EoE-related diagnoses, procedures and complications were identified over the 12-month baseline period + 30 days after the index date.
EoE, eosinophilic esophagitis; ED, emergency department.

Figure: Table 2. Demographics and clinical characteristics of patients stratified by EoE disease severity during the baseline period + 30 days after the index date.*
*Severity was classified based on EoE-related diagnoses, procedures and complications identified over the 12-month baseline period + 30 days after the index date.
†Patients with the answer ‘Yes’ to this question likely had established EoE (i.e. before the index date), whereas those with the answer ‘No’ were likely to be incident cases. Diagnosis was defined as a patient having ≥1 claim containing the diagnosis code for EoE (ICD-10-CM: K20.0).
‡For the purposes of the algorithm, patients qualifying with these procedures/diagnoses had to be aged ≥ 18 years.
§For the purposes of the algorithm, patients qualifying with these procedures/diagnoses had to be aged < 18 years.
CCI, Charlson Comorbidity Index; EoE, eosinophilic esophagitis; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; SD, standard deviation.
Disclosures:
Eric Shah: Ardelyx – Consultant. Mahana Therapeutics – Consultant. NeurAxis – Consultant. Salix Pharmaceuticals – Consultant. Takeda Pharmaceuticals – Consultant. University of Michigan – Intellectual Property/Patents.
Bridgett Goodwin: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Yiyan Liu: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Bertha de Los Santos: Takeda Pharmaceuticals USA, Inc. – Employee.
Siddhi Korgaonkar: RTI Health Solutions – Employee.
Juliana Meyers: RTI Health Solutions – Employee.
Carolyn Schaeffer-Koziol: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Brian Terreri: Takeda Pharmaceutical Company Limited – Stock-publicly held company(excluding mutual/index funds). Takeda Pharmaceuticals USA, Inc. – Employee.
Evan Dellon: AbbVie – Consultant. Adare/Ellodi – Consultant, Grant/Research Support. Akesobio – Consultant. Alfasigma – Consultant. ALK – Consultant. Allakos – Consultant, Grant/Research Support. Amgen – Consultant. Apogee – Consultant. Apollo – Consultant. Aqilion – Consultant, Grant/Research Support. Arena/Pfizer – Consultant, Grant/Research Support. Aslan – Consultant. AstraZeneca – Consultant, Grant/Research Support. Avir – Consultant. Biocryst – Consultant. Bryn – Consultant. Calypso – Consultant. Celgene/Receptos/Bristol Myers Squibb – Consultant, Grant/Research Support. Celldex – Consultant, Grant/Research Support. Dr. Falk Pharma – Consultant. EsoCap – Consultant. Eupraxia – Consultant, Grant/Research Support. Ferring – Consultant, Grant/Research Support. GI Reviewers – Consultant. GSK – Consultant, Grant/Research Support. Holoclara – Consultant, Grant/Research Support. Invea – Consultant, Grant/Research Support. Knightpoint – Consultant. LucidDx – Consultant. Meritage – Grant/Research Support. Miraca – Grant/Research Support. Morphic – Consultant. Nexstone Immunology/Uniquity – Consultant. Nutricia – Consultant, Grant/Research Support. Parexel/Calyx – Consultant. Phathom – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Revolo – Consultant, Grant/Research Support. Robarts/Alimentiv – Consultant. Sanofi – Consultant, Grant/Research Support. Shire/Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Third Harmonic Bio – Consultant. Uniquity – Grant/Research Support. Upstream Bio – Consultant.
Eric D. Shah, MD, MBA1, Bridgett Goodwin, PhD2, Yiyan Liu, PhD2, Bertha de Los Santos, PharmD3, Siddhi Korgaonkar, PhD4, Juliana Meyers, MA4, Carolyn R. Schaeffer-Koziol, PhD2, Brian Terreri, PharmD2, Evan S. Dellon, MD, MPH5. P0610 - Using Health Insurance Claims Data to Determine the Severity of Eosinophilic Esophagitis in Patients in the USA: A Retrospective Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
