Sunday Poster Session
Category: Esophagus
P0608 - Targeted Deprescribing of PPIs in Ambulatory Care: Outcomes From a Resident-Led Intervention
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Himsikhar Khataniar, MD (he/him/his)
Allegheny General Hospital
Pittsburgh, PA
Presenting Author(s)
Himsikhar Khataniar, MD1, Ikram Khaliq, MBBS2, Helene Bloom, DO3, Hany Habib, MD3, Kevin Taffe, MD, PhD2
1Allegheny General Hospital, Pittsburgh, PA; 2Allegheny Health Network, Pittsburgh, PA; 3Allegheny Health Network Medicine Institute, Pittsburgh, PA
Introduction: Chronic proton pump inhibitor (PPI) use—defined as therapy beyond 12 weeks without an ongoing indication—remains prevalent in ambulatory practice despite mounting evidence of associated long-term risks, including Clostridioides difficile infection, chronic kidney disease, and bone loss.
Methods: We designed and implemented a two-phase, 16-week Quality Improvement (QI) project at an academic resident clinic to reduce inappropriate PPI prescribing using a Plan–Do–Study–Act (PDSA) model. Phase 1 (Weeks 1–8) consisted of targeted educational sessions for resident and attending physicians on appropriate PPI use, adverse effects, and deprescribing strategies. Phase 2 (Weeks 9–16) involved a pharmacist-led chart review and de-escalation protocol in which eligible patients were advised to halve their dose for four weeks, followed by alternate-day dosing for two weeks, and then stop with optional antacid or H₂ blocker rescue. PPI order counts were tracked monthly from January 2024 to March 2025. Predictors of chronic PPI use were identified using multivariable logistic regression incorporating demographic and clinical comorbidity data.
Results: Over the 15-month period, 1,726 PPI orders were recorded, 99.7% of which were issued from the Fed-North clinic. Orders decreased from 144 in January 2024 to 49 in March 2025, reflecting a 66% relative reduction. The average monthly prescription count fell from 124 pre-intervention to 49 post-intervention. Logistic regression identified dyslipidemia (aOR 2.30; 95% CI 1.70–3.11), hypertension (aOR 1.71; 95% CI 1.22–2.40), and atrial fibrillation (aOR 1.62; 95% CI 1.05–2.49) as independent predictors of chronic PPI use. Male gender (aOR 0.73; 95% CI 0.56–0.95) and congestive heart failure (aOR 0.60; 95% CI 0.40–0.92) were associated with reduced odds. Race and ethnicity did not significantly predict chronic use.
Discussion: In conclusion, this QI initiative led to a sustained, meaningful reduction in inappropriate PPI use through a scalable, two-phase intervention. The pharmacist-driven taper protocol was well tolerated, with successful de-escalation in 78% of eligible patients and symptom recurrence in only 3.8%, all managed non-invasively. These findings support structured deprescribing workflows to optimize safe, evidence-based care in the outpatient setting.
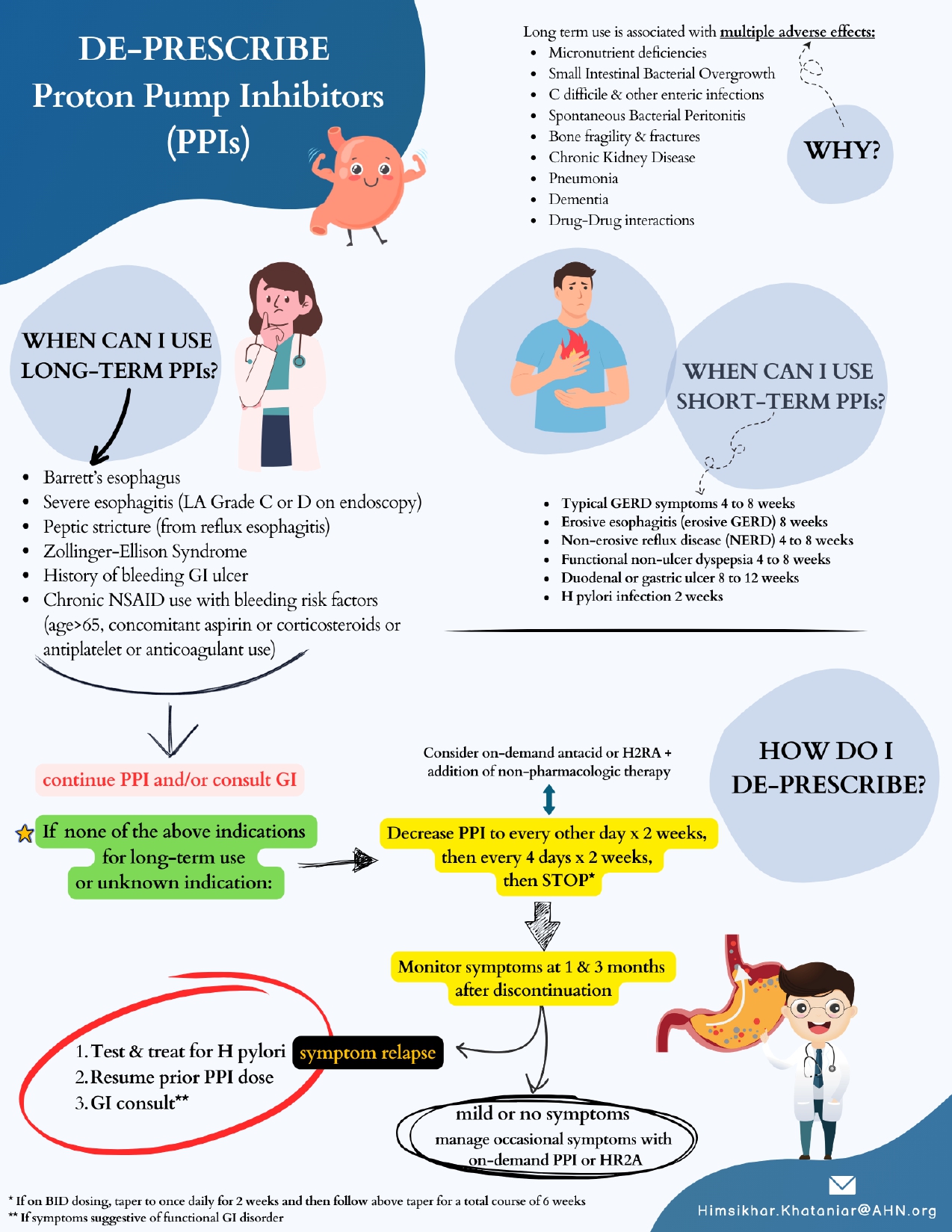
Figure: Figure 1: PPI Deprescribing Algorithm: A Quality Improvement Initiative - Flyer

Figure: Figure 2: Trends in Monthly PPI Prescribing Before and After QI Intervention
Disclosures:
Himsikhar Khataniar indicated no relevant financial relationships.
Ikram Khaliq indicated no relevant financial relationships.
Helene Bloom indicated no relevant financial relationships.
Hany Habib indicated no relevant financial relationships.
Kevin Taffe indicated no relevant financial relationships.
Himsikhar Khataniar, MD1, Ikram Khaliq, MBBS2, Helene Bloom, DO3, Hany Habib, MD3, Kevin Taffe, MD, PhD2. P0608 - Targeted Deprescribing of PPIs in Ambulatory Care: Outcomes From a Resident-Led Intervention, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Allegheny General Hospital, Pittsburgh, PA; 2Allegheny Health Network, Pittsburgh, PA; 3Allegheny Health Network Medicine Institute, Pittsburgh, PA
Introduction: Chronic proton pump inhibitor (PPI) use—defined as therapy beyond 12 weeks without an ongoing indication—remains prevalent in ambulatory practice despite mounting evidence of associated long-term risks, including Clostridioides difficile infection, chronic kidney disease, and bone loss.
Methods: We designed and implemented a two-phase, 16-week Quality Improvement (QI) project at an academic resident clinic to reduce inappropriate PPI prescribing using a Plan–Do–Study–Act (PDSA) model. Phase 1 (Weeks 1–8) consisted of targeted educational sessions for resident and attending physicians on appropriate PPI use, adverse effects, and deprescribing strategies. Phase 2 (Weeks 9–16) involved a pharmacist-led chart review and de-escalation protocol in which eligible patients were advised to halve their dose for four weeks, followed by alternate-day dosing for two weeks, and then stop with optional antacid or H₂ blocker rescue. PPI order counts were tracked monthly from January 2024 to March 2025. Predictors of chronic PPI use were identified using multivariable logistic regression incorporating demographic and clinical comorbidity data.
Results: Over the 15-month period, 1,726 PPI orders were recorded, 99.7% of which were issued from the Fed-North clinic. Orders decreased from 144 in January 2024 to 49 in March 2025, reflecting a 66% relative reduction. The average monthly prescription count fell from 124 pre-intervention to 49 post-intervention. Logistic regression identified dyslipidemia (aOR 2.30; 95% CI 1.70–3.11), hypertension (aOR 1.71; 95% CI 1.22–2.40), and atrial fibrillation (aOR 1.62; 95% CI 1.05–2.49) as independent predictors of chronic PPI use. Male gender (aOR 0.73; 95% CI 0.56–0.95) and congestive heart failure (aOR 0.60; 95% CI 0.40–0.92) were associated with reduced odds. Race and ethnicity did not significantly predict chronic use.
Discussion: In conclusion, this QI initiative led to a sustained, meaningful reduction in inappropriate PPI use through a scalable, two-phase intervention. The pharmacist-driven taper protocol was well tolerated, with successful de-escalation in 78% of eligible patients and symptom recurrence in only 3.8%, all managed non-invasively. These findings support structured deprescribing workflows to optimize safe, evidence-based care in the outpatient setting.

Figure: Figure 1: PPI Deprescribing Algorithm: A Quality Improvement Initiative - Flyer

Figure: Figure 2: Trends in Monthly PPI Prescribing Before and After QI Intervention
Disclosures:
Himsikhar Khataniar indicated no relevant financial relationships.
Ikram Khaliq indicated no relevant financial relationships.
Helene Bloom indicated no relevant financial relationships.
Hany Habib indicated no relevant financial relationships.
Kevin Taffe indicated no relevant financial relationships.
Himsikhar Khataniar, MD1, Ikram Khaliq, MBBS2, Helene Bloom, DO3, Hany Habib, MD3, Kevin Taffe, MD, PhD2. P0608 - Targeted Deprescribing of PPIs in Ambulatory Care: Outcomes From a Resident-Led Intervention, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
