Sunday Poster Session
Category: IBD
P1040 - Accessing Escalated Risankizumab in Crohn’s Disease: How Long Does It Take?
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Patrick Nichols, PharmD (he/him/his)
Vanderbilt Inflammatory Bowel Disease Clinic
Nashville, TN
Presenting Author(s)
Patrick Nichols, PharmD1, Montana Gunter, 2, Autumn D.. Zuckerman, PharmD2, Nicolas Gargurevich, MS2, Robin L. Dalal, MD1
1Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 2Vanderbilt University Medical Center, Nashville, TN
Introduction: Risankizumab (RZB) doses above 360mg or 180mg subcutaneous (SC) injection every 8 weeks are not FDA-approved for Crohn’s disease (CD) but may improve disease control. Escalated doses can be challenging to access from payors or manufacturer patient assistance programs (PAP), though the process has not been well described.
Methods: A retrospective observational single-center cohort study evaluated patients with CD who had at least one escalated dose of RZB between June 2022 and April 2024. Outcomes included time from escalated dosing referral to medication coverage (via insurance approval or manufacturer assistance) and method of medication coverage. Descriptive statistics summarized continuous variables and categorical variables (Median (IQR) or Mean (SD) and frequencies as percents respectively). A multivariable Cox regression model evaluated time from referral to coverage controlling for age, sex, insurance type, and number of previous therapies tried.
Results: In the 63 patients included, over half (57%) were female, mostly white (94%), with an average disease duration of 17 years (SD 11), with mostly commercial insurance (75%). All patients received the 360mg dose; 51 (81%) were escalated to every 6 weeks and 13 (21%) were escalated to every 4 weeks. Patients had tried and failed a median of 4 (IQR 2, 4.5) previous therapies. The median time from referral to coverage was 14 days (IQR 3, 36). Patients were approved via no prior authorization (PA) required (32%), PA (24%), first level appeal (16%), second level appeal/external review/peer to peer (22%), or manufacturer PAP (6%). Time to coverage increased with each additional step required, median time was 6 days (IQR 1, 14) if no PA needed, 10 days (IQR 3, 18) if PA needed, 32 days (IQR 14, 60) if first level appeal needed, 43 days (IQR 35, 61) if second level appeal/external review/peer to peer needed, and 0 days for PAP (all 4 patients were enrolled pre-escalation). Patients with government insurance and patients who had tried and failed more therapies were more likely to receive coverage faster (HR 1.93, p=0.039 and HR 1.2, p=0.018, respectively) (Figure 1).
Discussion: In patients who received at least one dose of escalated RZB, accessing escalated RZB dosing took about 2 weeks but often up to a month. The lengthy access process often required steps beyond a PA, highlighting the difficulties faced by patients who require escalation to achieve clinical remission.
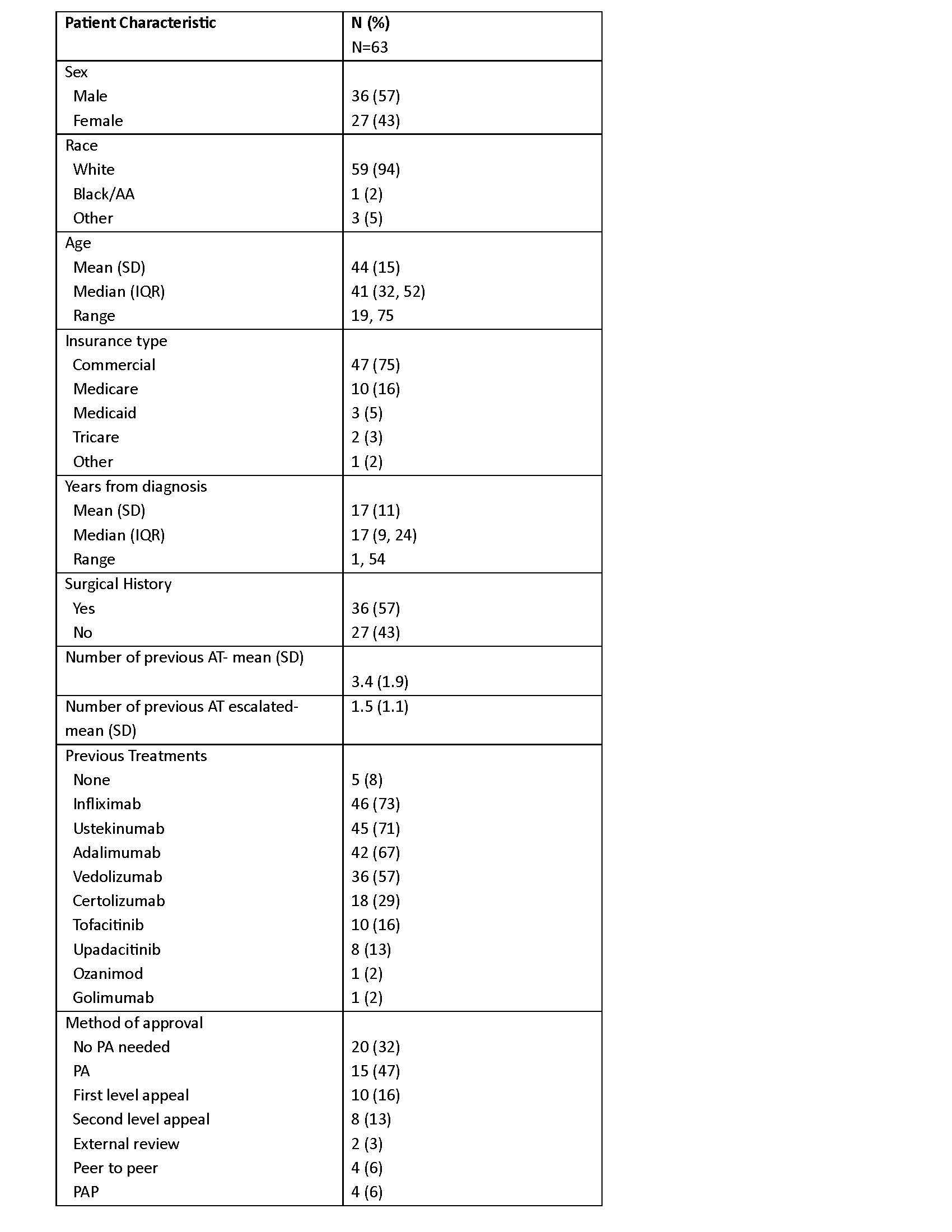
Figure: Table shows characteristics of 63 patients included in study. Abbreviations: AA: African American, AT: advanced therapy, SD: standard deviation, IQR: interquartile range, PA: Prior Authorization, PAP: Patient Assistance Program

Figure: Figure 1 Caption. The probability of not having approval at any time is shown by commercial vs. Government insurance. Patients with government insurance received coverage faster than commercial patients (HR 1.93, p=0.039.)
Disclosures:
Patrick Nichols: Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly and Company – Speakers Bureau. Johnson & Johnson Innovative Medicine – Advisory Committee/Board Member.
Montana Gunter indicated no relevant financial relationships.
Autumn Zuckerman: AstraZeneca – Grant/Research Support. Beigene – Grant/Research Support. Pfizer Inc. – Grant/Research Support. Sanofi Inc – Grant/Research Support. UCB – Grant/Research Support.
Nicolas Gargurevich indicated no relevant financial relationships.
Robin Dalal: AbbVie – Consultant.
Patrick Nichols, PharmD1, Montana Gunter, 2, Autumn D.. Zuckerman, PharmD2, Nicolas Gargurevich, MS2, Robin L. Dalal, MD1. P1040 - Accessing Escalated Risankizumab in Crohn’s Disease: How Long Does It Take?, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 2Vanderbilt University Medical Center, Nashville, TN
Introduction: Risankizumab (RZB) doses above 360mg or 180mg subcutaneous (SC) injection every 8 weeks are not FDA-approved for Crohn’s disease (CD) but may improve disease control. Escalated doses can be challenging to access from payors or manufacturer patient assistance programs (PAP), though the process has not been well described.
Methods: A retrospective observational single-center cohort study evaluated patients with CD who had at least one escalated dose of RZB between June 2022 and April 2024. Outcomes included time from escalated dosing referral to medication coverage (via insurance approval or manufacturer assistance) and method of medication coverage. Descriptive statistics summarized continuous variables and categorical variables (Median (IQR) or Mean (SD) and frequencies as percents respectively). A multivariable Cox regression model evaluated time from referral to coverage controlling for age, sex, insurance type, and number of previous therapies tried.
Results: In the 63 patients included, over half (57%) were female, mostly white (94%), with an average disease duration of 17 years (SD 11), with mostly commercial insurance (75%). All patients received the 360mg dose; 51 (81%) were escalated to every 6 weeks and 13 (21%) were escalated to every 4 weeks. Patients had tried and failed a median of 4 (IQR 2, 4.5) previous therapies. The median time from referral to coverage was 14 days (IQR 3, 36). Patients were approved via no prior authorization (PA) required (32%), PA (24%), first level appeal (16%), second level appeal/external review/peer to peer (22%), or manufacturer PAP (6%). Time to coverage increased with each additional step required, median time was 6 days (IQR 1, 14) if no PA needed, 10 days (IQR 3, 18) if PA needed, 32 days (IQR 14, 60) if first level appeal needed, 43 days (IQR 35, 61) if second level appeal/external review/peer to peer needed, and 0 days for PAP (all 4 patients were enrolled pre-escalation). Patients with government insurance and patients who had tried and failed more therapies were more likely to receive coverage faster (HR 1.93, p=0.039 and HR 1.2, p=0.018, respectively) (Figure 1).
Discussion: In patients who received at least one dose of escalated RZB, accessing escalated RZB dosing took about 2 weeks but often up to a month. The lengthy access process often required steps beyond a PA, highlighting the difficulties faced by patients who require escalation to achieve clinical remission.

Figure: Table shows characteristics of 63 patients included in study. Abbreviations: AA: African American, AT: advanced therapy, SD: standard deviation, IQR: interquartile range, PA: Prior Authorization, PAP: Patient Assistance Program

Figure: Figure 1 Caption. The probability of not having approval at any time is shown by commercial vs. Government insurance. Patients with government insurance received coverage faster than commercial patients (HR 1.93, p=0.039.)
Disclosures:
Patrick Nichols: Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly and Company – Speakers Bureau. Johnson & Johnson Innovative Medicine – Advisory Committee/Board Member.
Montana Gunter indicated no relevant financial relationships.
Autumn Zuckerman: AstraZeneca – Grant/Research Support. Beigene – Grant/Research Support. Pfizer Inc. – Grant/Research Support. Sanofi Inc – Grant/Research Support. UCB – Grant/Research Support.
Nicolas Gargurevich indicated no relevant financial relationships.
Robin Dalal: AbbVie – Consultant.
Patrick Nichols, PharmD1, Montana Gunter, 2, Autumn D.. Zuckerman, PharmD2, Nicolas Gargurevich, MS2, Robin L. Dalal, MD1. P1040 - Accessing Escalated Risankizumab in Crohn’s Disease: How Long Does It Take?, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
