Sunday Poster Session
Category: IBD
P1039 - Treatment Outcomes of Risankizumab Dose Escalation in Crohn’s Disease
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Patrick Nichols, PharmD (he/him/his)
Vanderbilt Inflammatory Bowel Disease Clinic
Nashville, TN
Presenting Author(s)
Award: ACG Presidential Poster Award
Patrick Nichols, PharmD1, Montana Gunter, 2, Autumn D.. Zuckerman, PharmD2, Nicolas Gargurevich, MS2, Robin L. Dalal, MD1
1Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 2Vanderbilt University Medical Center, Nashville, TN
Introduction: Limited data exists evaluating outcomes of dosing risankizumab (RZB) above the FDA-approved maintenance dose (360mg or 180mg subcutaneous every 8 weeks) for the treatment of Crohn’s disease (CD).
Methods: A retrospective observational single-center cohort study evaluated patients with CD who received at least one escalated dose of RZB between June 2022 and April 2024. Patients without follow-up within 8 months post-escalation were excluded from the analysis of follow-up outcomes. Outcomes included response to therapy (either endoscopic or patient-reported symptom improvement), medication changes, and changes in clinical measures (ESR, CRP, HBI, and SIBDQ) within 8 months of escalation. Descriptive statistics summarized continuous variables and categorical variables (median [IQR] or mean [SD] and frequencies as percents respectively). A multivariable logistic regression model evaluated patient improvement at any point within 8 months of escalation controlling for days from escalation to last evaluation in the study period, years since diagnosis, and number of previous treatments tried.
Results: In the 63 included patients, over half (57%) were female, mostly white (94%), with an average disease duration of 17 years (SD ±11). Thirty-two patients (51%) were escalated due to symptoms and 28 (44%) were escalated due to endoscopic findings. All 63 patients received the 360mg dose; 50 (79%) were escalated to every 6 weeks and 13 (21%) to every 4 weeks. Patients had tried and failed an average of 4 (IQR 2, 4.5) previous therapies. The median number of on-body injection doses before the decision to escalate was 3 (IQR 2, 3.5). Of 58 patients with a follow-up within 8 months of escalation, median last evaluation occurred at 157 (IQR 107, 180) days. Almost half of patients improved (n=27, 47%), others remained stable (n=15, 26%) or worsened (n=16, 28%). Patients who did not respond had often previously failed multiple advanced therapies (Figure 1). No covariates in the multivariate regression analysis were significant. For the entire cohort, 34% of patients had additional medication changes within 8 months of escalation. With available data, improvements were seen in CRP (54%), ESR (46%), HBI (57%), and SIBDQ (60%) measures.
Discussion: In a heavily treatment experienced population of patients with CD, almost half had a clinical response to escalated dosing of RZB within 8 months. Patients who have previously failed numerous treatments may be more likely to not respond to RZB dose escalation.
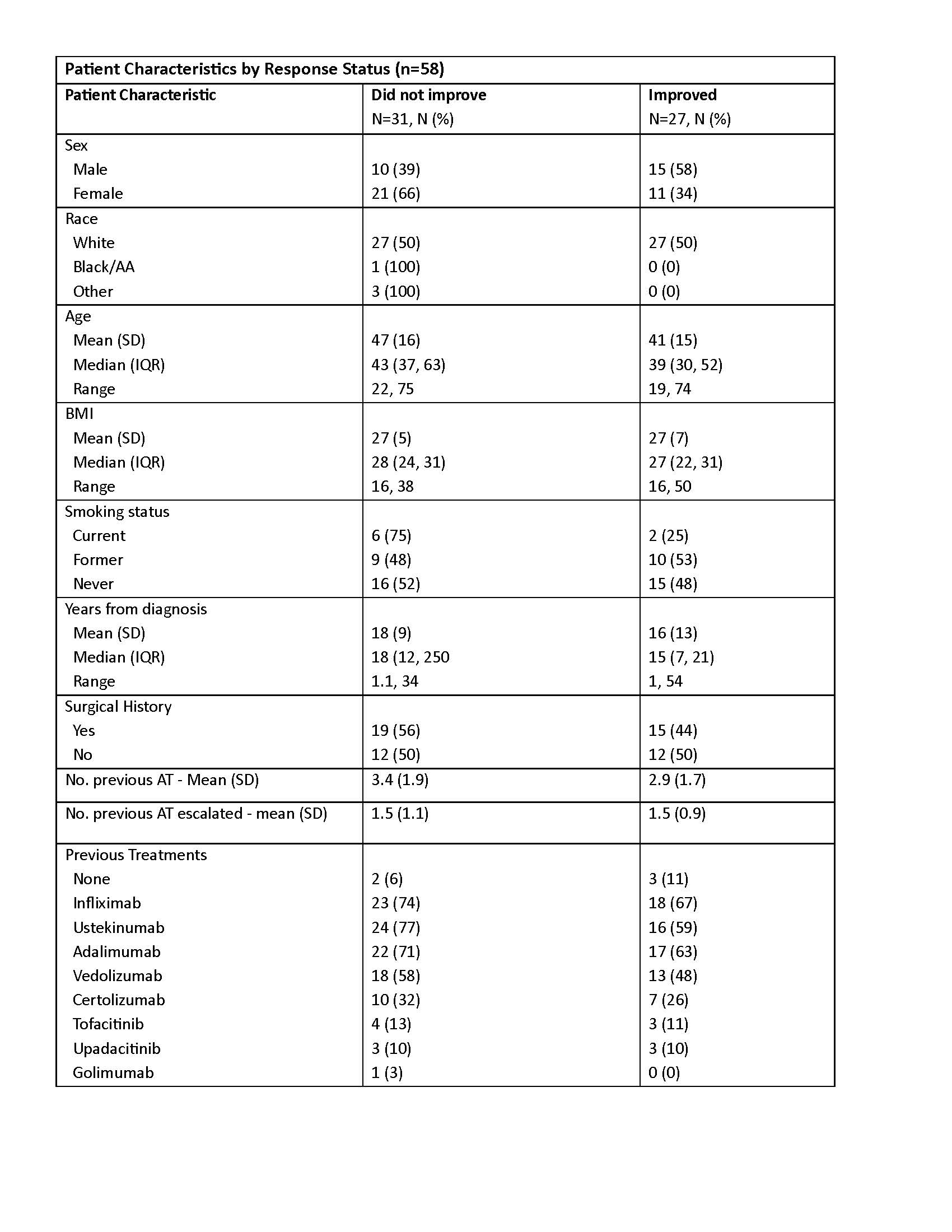
Figure: Table shows characteristics of the 58 patients with 8-month follow-up
Abbreviations: AA: African American, SD: standard deviation, IQR: interquartile range, AT: advanced therapy

Figure: Figure 1 Caption. Patient response (improved, stable, worsened) within 8 months of escalation is shown by the number of previous advanced therapies tried and failed. In the 27 (n=27/58, 46%) patients that improved, 3 (11%) had not tried previous therapy, 9 (33%) had tried 1-2, 11 (41%) had tried 3-4, and 4 (15%) had tried 5+. In the 16 (28%) of patients that worsened, 1 (6%) had not tried previous therapy, 4 (25%) had tried 1-2, 6 (38%) had tried 3-4, and 5 (31%) had tried 5+.
Disclosures:
Patrick Nichols: Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly and Company – Speakers Bureau. Johnson & Johnson Innovative Medicine – Advisory Committee/Board Member.
Montana Gunter indicated no relevant financial relationships.
Autumn Zuckerman: AstraZeneca – Grant/Research Support. Beigene – Grant/Research Support. Pfizer Inc. – Grant/Research Support. Sanofi Inc – Grant/Research Support. UCB – Grant/Research Support.
Nicolas Gargurevich indicated no relevant financial relationships.
Robin Dalal: AbbVie – Consultant.
Patrick Nichols, PharmD1, Montana Gunter, 2, Autumn D.. Zuckerman, PharmD2, Nicolas Gargurevich, MS2, Robin L. Dalal, MD1. P1039 - Treatment Outcomes of Risankizumab Dose Escalation in Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Patrick Nichols, PharmD1, Montana Gunter, 2, Autumn D.. Zuckerman, PharmD2, Nicolas Gargurevich, MS2, Robin L. Dalal, MD1
1Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 2Vanderbilt University Medical Center, Nashville, TN
Introduction: Limited data exists evaluating outcomes of dosing risankizumab (RZB) above the FDA-approved maintenance dose (360mg or 180mg subcutaneous every 8 weeks) for the treatment of Crohn’s disease (CD).
Methods: A retrospective observational single-center cohort study evaluated patients with CD who received at least one escalated dose of RZB between June 2022 and April 2024. Patients without follow-up within 8 months post-escalation were excluded from the analysis of follow-up outcomes. Outcomes included response to therapy (either endoscopic or patient-reported symptom improvement), medication changes, and changes in clinical measures (ESR, CRP, HBI, and SIBDQ) within 8 months of escalation. Descriptive statistics summarized continuous variables and categorical variables (median [IQR] or mean [SD] and frequencies as percents respectively). A multivariable logistic regression model evaluated patient improvement at any point within 8 months of escalation controlling for days from escalation to last evaluation in the study period, years since diagnosis, and number of previous treatments tried.
Results: In the 63 included patients, over half (57%) were female, mostly white (94%), with an average disease duration of 17 years (SD ±11). Thirty-two patients (51%) were escalated due to symptoms and 28 (44%) were escalated due to endoscopic findings. All 63 patients received the 360mg dose; 50 (79%) were escalated to every 6 weeks and 13 (21%) to every 4 weeks. Patients had tried and failed an average of 4 (IQR 2, 4.5) previous therapies. The median number of on-body injection doses before the decision to escalate was 3 (IQR 2, 3.5). Of 58 patients with a follow-up within 8 months of escalation, median last evaluation occurred at 157 (IQR 107, 180) days. Almost half of patients improved (n=27, 47%), others remained stable (n=15, 26%) or worsened (n=16, 28%). Patients who did not respond had often previously failed multiple advanced therapies (Figure 1). No covariates in the multivariate regression analysis were significant. For the entire cohort, 34% of patients had additional medication changes within 8 months of escalation. With available data, improvements were seen in CRP (54%), ESR (46%), HBI (57%), and SIBDQ (60%) measures.
Discussion: In a heavily treatment experienced population of patients with CD, almost half had a clinical response to escalated dosing of RZB within 8 months. Patients who have previously failed numerous treatments may be more likely to not respond to RZB dose escalation.

Figure: Table shows characteristics of the 58 patients with 8-month follow-up
Abbreviations: AA: African American, SD: standard deviation, IQR: interquartile range, AT: advanced therapy

Figure: Figure 1 Caption. Patient response (improved, stable, worsened) within 8 months of escalation is shown by the number of previous advanced therapies tried and failed. In the 27 (n=27/58, 46%) patients that improved, 3 (11%) had not tried previous therapy, 9 (33%) had tried 1-2, 11 (41%) had tried 3-4, and 4 (15%) had tried 5+. In the 16 (28%) of patients that worsened, 1 (6%) had not tried previous therapy, 4 (25%) had tried 1-2, 6 (38%) had tried 3-4, and 5 (31%) had tried 5+.
Disclosures:
Patrick Nichols: Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly and Company – Speakers Bureau. Johnson & Johnson Innovative Medicine – Advisory Committee/Board Member.
Montana Gunter indicated no relevant financial relationships.
Autumn Zuckerman: AstraZeneca – Grant/Research Support. Beigene – Grant/Research Support. Pfizer Inc. – Grant/Research Support. Sanofi Inc – Grant/Research Support. UCB – Grant/Research Support.
Nicolas Gargurevich indicated no relevant financial relationships.
Robin Dalal: AbbVie – Consultant.
Patrick Nichols, PharmD1, Montana Gunter, 2, Autumn D.. Zuckerman, PharmD2, Nicolas Gargurevich, MS2, Robin L. Dalal, MD1. P1039 - Treatment Outcomes of Risankizumab Dose Escalation in Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

