Sunday Poster Session
Category: IBD
P1087 - Medical Cannabis for IBD: Differential Responses to Cannabinoids in IL-10 Deficient Model of IBD
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Ivanna Morejon, BS (she/her/hers)
Lewis Katz School of Medicine at Temple University
Philadelphia, PA
Presenting Author(s)
Ivanna Morejon, BS, Ana M. Gamero, PhD, Amy Alvarado, BS, Sara J. Ward, PhD
Lewis Katz School of Medicine at Temple University, Philadelphia, PA
Introduction: Inflammatory bowel disease (IBD) is a chronic, relapsing GI condition that remains difficult to treat. Many patients experience limited benefit from standard therapies, steroid dependence, or require surgery. The endocannabinoid system, which modulates gut immune and epithelial function, is an emerging therapeutic target. Cannabinoids like Δ9-THC, CBD, and β-caryophyllene (BCP) show anti-inflammatory effects in acute colitis models, but clinical use is limited by inconsistent efficacy, psychotropic effects, and unstandardized dosing. The IL-10 knockout (IL-10 KO) mouse, a chronic, immune-mediated colitis model, offers a platform to assess long-term cannabinoid efficacy. This study evaluates the individual and combined effects of THC, CBD, and BCP in IL-10 KO mice, with attention to sex- and compound-specific long-term responses.
Methods: Groups of IL-10 KO mice (n=5–8/sex) were treated intraperitoneally 3x/week starting at 4 weeks of age. Treatment groups received vehicle, THC (20 mg/kg), CBD (20 mg/kg), BCP (30 mg/kg), or a combination of THC+CBD (20+20 mg/kg). Disease progression was monitored visually and confirmed post-mortem. At 18 weeks, colons were harvested for histological evaluation using H&E and Alcian Blue staining and scored for epithelial integrity and inflammation. Wild-type mice given single compounds served as controls. Additional groups receiving BCP (10 mg/kg), CBD (100 mg/kg), and THC+BCP (20+30 mg/kg) are currently under investigation.
Results: THC treatment reduced colitis severity in IL-10 KO females, while CBD showed modest efficacy in males only. BCP showed the most robust protective effects in both sexes with notable preservation of mucosal integrity. Interestingly, the THC+CBD combination did not produce the expected synergistic effect and offered little benefit compared to either compound alone, suggesting potential antagonistic interaction. No adverse effects were observed in wild-type mice.
Discussion: Cannabinoid treatments showed distinct sex- and compound-specific effects. BCP was the most consistently effective, likely via CB2 receptor agonism. Limited CBD efficacy in females may reflect faster metabolic clearance; a higher dose is now being tested. The antagonism seen with THC+CBD highlights the complexity of cannabinoid interactions and the need for careful optimization of combination therapies. Overall, these findings support continued development of cannabinoid-based therapies for IBD, including dosing and long-term remission evaluation.
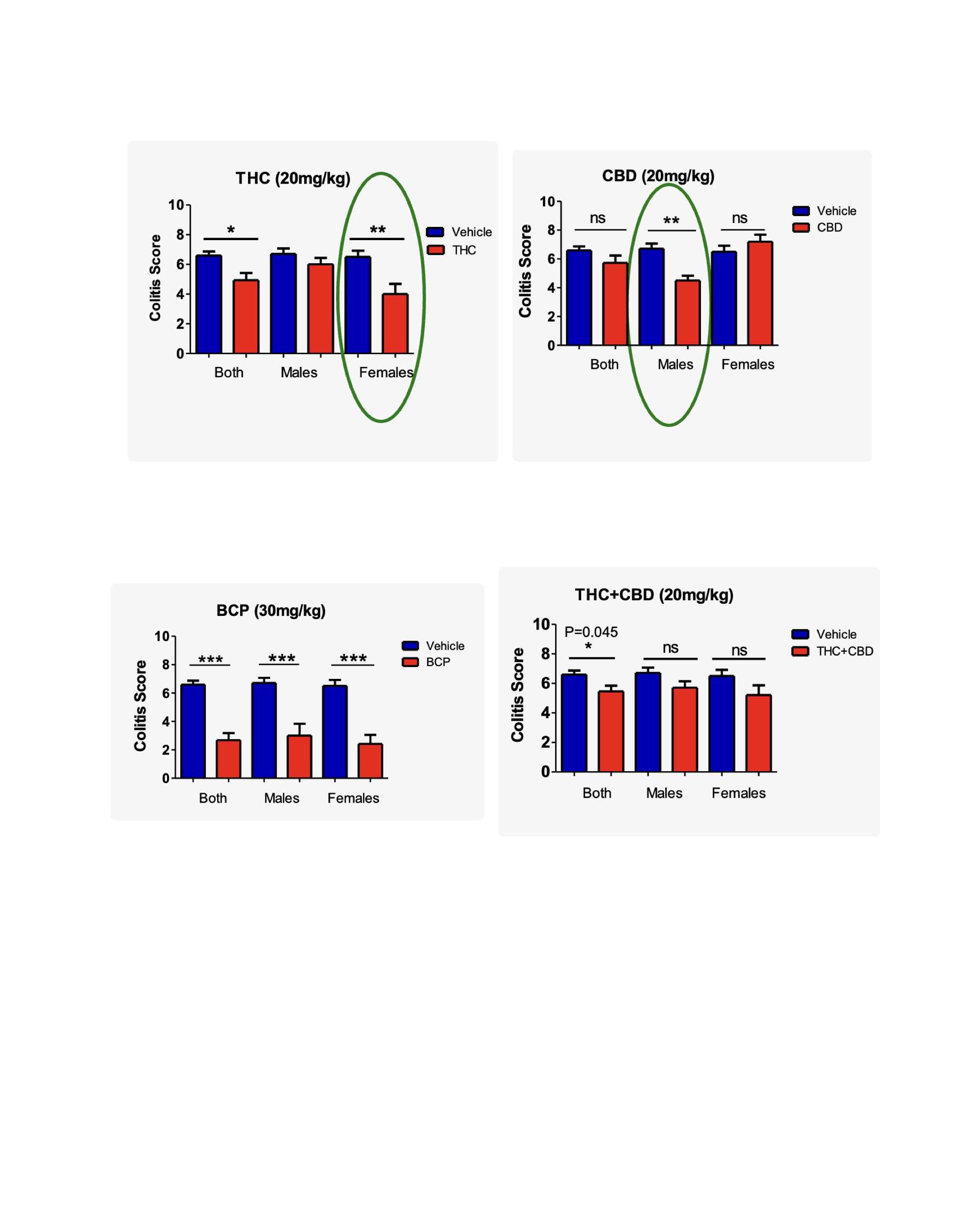
Figure: Figure 1. Sex-specific effects of cannabinoid treatment on colitis severity in IL-10 KO mice. Colitis scores at 18 weeks are stratified by sex. THC (20 mg/kg) significantly reduced scores in females (p < 0.01) and overall (*p < 0.05), with no significant effect in males. CBD (20 mg/kg) was effective only in males (p < 0.01), with no benefit in females. BCP (30 mg/kg) significantly reduced colitis scores in both sexes and overall (*p < 0.001). The THC+CBD combination (20+20 mg/kg) showed modest overall benefit (p = 0.045) but no significant sex-specific effects.
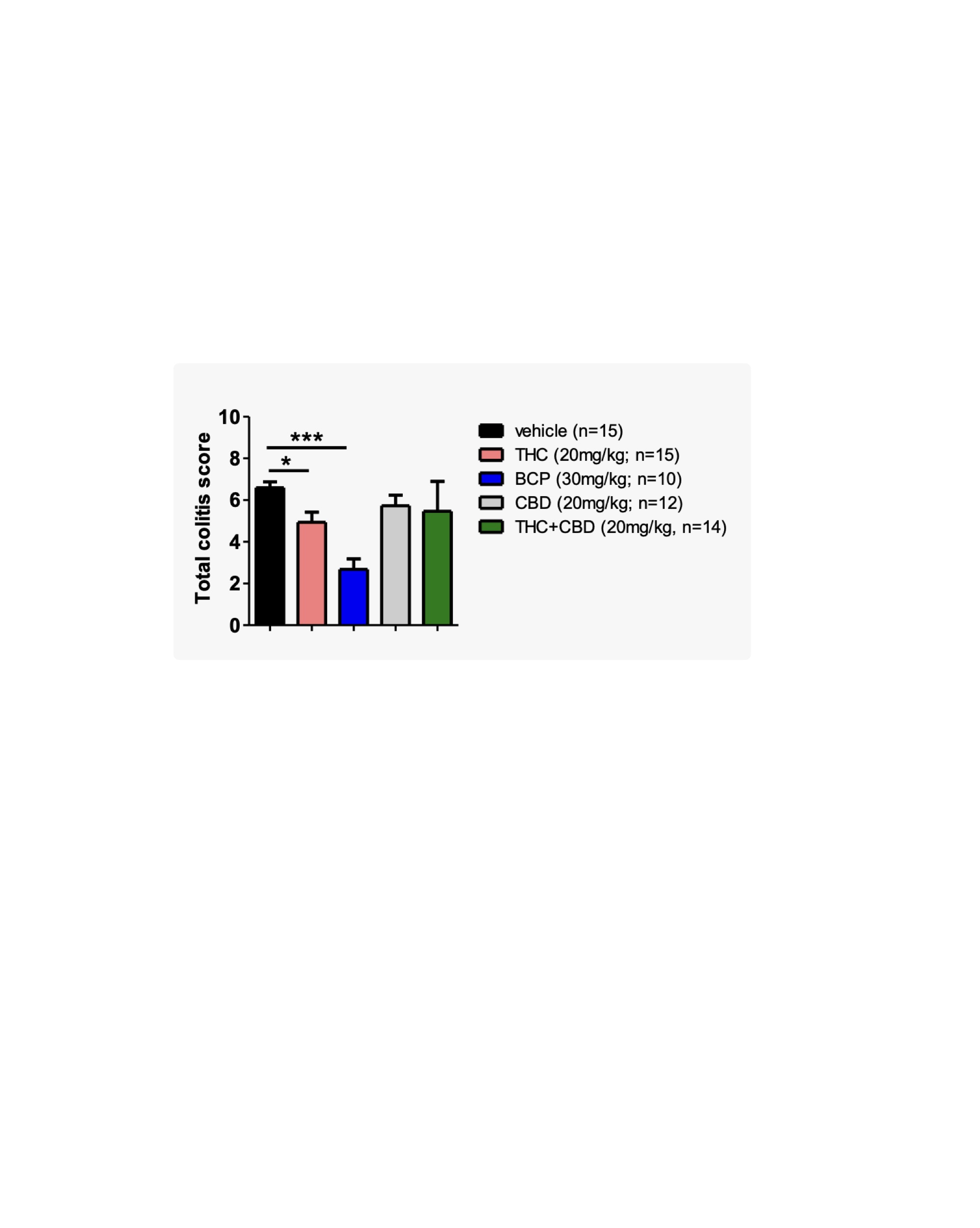
Figure: Figure 2. Cannabinoid treatment reduces colitis severity in IL-10 KO mice. Total colitis scores at 18 weeks show that BCP (30 mg/kg) significantly reduced disease severity compared to vehicle (*p < 0.05, ***p < 0.001), with THC (20 mg/kg) also showing moderate benefit. CBD (20 mg/kg) and THC+CBD (20+20 mg/kg) treatments showed no significant improvement.
Disclosures:
Ivanna Morejon indicated no relevant financial relationships.
Ana Gamero indicated no relevant financial relationships.
Amy Alvarado indicated no relevant financial relationships.
Sara Ward indicated no relevant financial relationships.
Ivanna Morejon, BS, Ana M. Gamero, PhD, Amy Alvarado, BS, Sara J. Ward, PhD. P1087 - Medical Cannabis for IBD: Differential Responses to Cannabinoids in IL-10 Deficient Model of IBD, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Lewis Katz School of Medicine at Temple University, Philadelphia, PA
Introduction: Inflammatory bowel disease (IBD) is a chronic, relapsing GI condition that remains difficult to treat. Many patients experience limited benefit from standard therapies, steroid dependence, or require surgery. The endocannabinoid system, which modulates gut immune and epithelial function, is an emerging therapeutic target. Cannabinoids like Δ9-THC, CBD, and β-caryophyllene (BCP) show anti-inflammatory effects in acute colitis models, but clinical use is limited by inconsistent efficacy, psychotropic effects, and unstandardized dosing. The IL-10 knockout (IL-10 KO) mouse, a chronic, immune-mediated colitis model, offers a platform to assess long-term cannabinoid efficacy. This study evaluates the individual and combined effects of THC, CBD, and BCP in IL-10 KO mice, with attention to sex- and compound-specific long-term responses.
Methods: Groups of IL-10 KO mice (n=5–8/sex) were treated intraperitoneally 3x/week starting at 4 weeks of age. Treatment groups received vehicle, THC (20 mg/kg), CBD (20 mg/kg), BCP (30 mg/kg), or a combination of THC+CBD (20+20 mg/kg). Disease progression was monitored visually and confirmed post-mortem. At 18 weeks, colons were harvested for histological evaluation using H&E and Alcian Blue staining and scored for epithelial integrity and inflammation. Wild-type mice given single compounds served as controls. Additional groups receiving BCP (10 mg/kg), CBD (100 mg/kg), and THC+BCP (20+30 mg/kg) are currently under investigation.
Results: THC treatment reduced colitis severity in IL-10 KO females, while CBD showed modest efficacy in males only. BCP showed the most robust protective effects in both sexes with notable preservation of mucosal integrity. Interestingly, the THC+CBD combination did not produce the expected synergistic effect and offered little benefit compared to either compound alone, suggesting potential antagonistic interaction. No adverse effects were observed in wild-type mice.
Discussion: Cannabinoid treatments showed distinct sex- and compound-specific effects. BCP was the most consistently effective, likely via CB2 receptor agonism. Limited CBD efficacy in females may reflect faster metabolic clearance; a higher dose is now being tested. The antagonism seen with THC+CBD highlights the complexity of cannabinoid interactions and the need for careful optimization of combination therapies. Overall, these findings support continued development of cannabinoid-based therapies for IBD, including dosing and long-term remission evaluation.

Figure: Figure 1. Sex-specific effects of cannabinoid treatment on colitis severity in IL-10 KO mice. Colitis scores at 18 weeks are stratified by sex. THC (20 mg/kg) significantly reduced scores in females (p < 0.01) and overall (*p < 0.05), with no significant effect in males. CBD (20 mg/kg) was effective only in males (p < 0.01), with no benefit in females. BCP (30 mg/kg) significantly reduced colitis scores in both sexes and overall (*p < 0.001). The THC+CBD combination (20+20 mg/kg) showed modest overall benefit (p = 0.045) but no significant sex-specific effects.

Figure: Figure 2. Cannabinoid treatment reduces colitis severity in IL-10 KO mice. Total colitis scores at 18 weeks show that BCP (30 mg/kg) significantly reduced disease severity compared to vehicle (*p < 0.05, ***p < 0.001), with THC (20 mg/kg) also showing moderate benefit. CBD (20 mg/kg) and THC+CBD (20+20 mg/kg) treatments showed no significant improvement.
Disclosures:
Ivanna Morejon indicated no relevant financial relationships.
Ana Gamero indicated no relevant financial relationships.
Amy Alvarado indicated no relevant financial relationships.
Sara Ward indicated no relevant financial relationships.
Ivanna Morejon, BS, Ana M. Gamero, PhD, Amy Alvarado, BS, Sara J. Ward, PhD. P1087 - Medical Cannabis for IBD: Differential Responses to Cannabinoids in IL-10 Deficient Model of IBD, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
