Sunday Poster Session
Category: Interventional Endoscopy
P1463 - Use of a Novel Electrosurgical Cystotome for Biliary Stricture Dilation
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Hadi Khaled Abou Zeid, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Rishad Khan, MD1, Barham Abu Dayyeh, MD, MPH2, Ryan Law, DO1, Andrew Storm, MD1
1Mayo Clinic, Rochester, MN; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: The endoscopic management of severe biliary strictures presents a significant clinical challenge, particularly when standard dilators fail to access or provide adequate dilation. A new endoscopic ultrasound (EUS)-guided cystotome has recently emerged as an electrosurgically assisted tool for access procedures. In this study, we aim to assess the safety and efficacy of the cystotome for Endoscopic Retrograde Cholangiopancreatography (ERCP)-guided stricture dilation.
Case Description/
Methods: Three patients (mean age 67, range of 51-89, 2 males) underwent stricturotomy using the cystotome after failure of conventional biliary balloon dilation (100%) (Table1). Two patients were undergoing ERCP for gallbladder stenting due to acute cholecystitis, which resulted in stricture of the cystic duct, while the third was being followed up for biliary sclerosis resulting in a stricture of a right posterior intrahepatic duct. Technical success was defined by successful stricture dilation and stent placement. Clinical success was defined as resolution of symptoms post-procedure. No periprocedural complications were reported. The procedure achieved both technical and clinical success rates of 100%. During the follow-up period, there were no signs of recurrence, long-term complications, or need for further interventions.
Discussion: This pilot cohort suggests that the EUS-guided cystotome is a safe and effective alternative for dilation of challenging biliary strictures during ERCP, including both cystic duct and intrahepatic duct strictures. Additional studies may validate these results and provide stronger evidence for use in clinical ERCP practice.
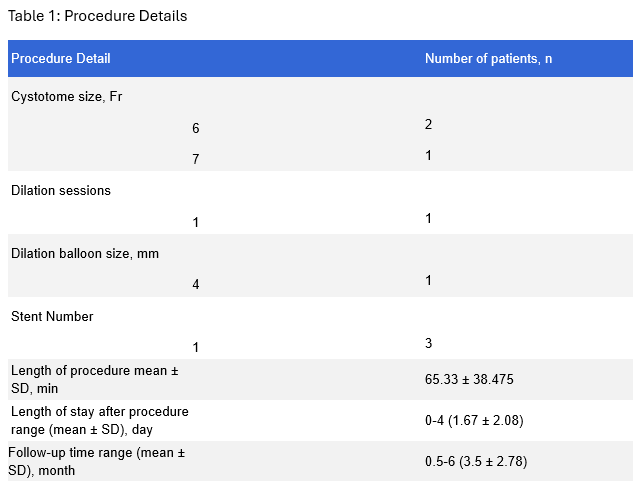
Figure: Table 1: Procedure Details
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
Yara Salameh indicated no relevant financial relationships.
Rishad Khan indicated no relevant financial relationships.
Barham Abu Dayyeh: Boston Scientific, Medtronic, Apollo Endosurgery, and Olympus – Consultant. Boston Scientific, Medtronic, Apollo Endosurgery, and USGI Medical – Grant/Research Support. Endogenex technology licensed by Mayo Clinic, with institutional equity and royalty through Mayo Clinic's invention policy. – coinventor.
Ryan Law: Boston Scientific – Consultant, Grant/Research Support. Neptune Medical – Data safety monitoring board. Olympus America – Consultant, Grant/Research Support. UpToDate – Royalties.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Rishad Khan, MD1, Barham Abu Dayyeh, MD, MPH2, Ryan Law, DO1, Andrew Storm, MD1. P1463 - Use of a Novel Electrosurgical Cystotome for Biliary Stricture Dilation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: The endoscopic management of severe biliary strictures presents a significant clinical challenge, particularly when standard dilators fail to access or provide adequate dilation. A new endoscopic ultrasound (EUS)-guided cystotome has recently emerged as an electrosurgically assisted tool for access procedures. In this study, we aim to assess the safety and efficacy of the cystotome for Endoscopic Retrograde Cholangiopancreatography (ERCP)-guided stricture dilation.
Case Description/
Methods: Three patients (mean age 67, range of 51-89, 2 males) underwent stricturotomy using the cystotome after failure of conventional biliary balloon dilation (100%) (Table1). Two patients were undergoing ERCP for gallbladder stenting due to acute cholecystitis, which resulted in stricture of the cystic duct, while the third was being followed up for biliary sclerosis resulting in a stricture of a right posterior intrahepatic duct. Technical success was defined by successful stricture dilation and stent placement. Clinical success was defined as resolution of symptoms post-procedure. No periprocedural complications were reported. The procedure achieved both technical and clinical success rates of 100%. During the follow-up period, there were no signs of recurrence, long-term complications, or need for further interventions.
Discussion: This pilot cohort suggests that the EUS-guided cystotome is a safe and effective alternative for dilation of challenging biliary strictures during ERCP, including both cystic duct and intrahepatic duct strictures. Additional studies may validate these results and provide stronger evidence for use in clinical ERCP practice.

Figure: Table 1: Procedure Details
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
Yara Salameh indicated no relevant financial relationships.
Rishad Khan indicated no relevant financial relationships.
Barham Abu Dayyeh: Boston Scientific, Medtronic, Apollo Endosurgery, and Olympus – Consultant. Boston Scientific, Medtronic, Apollo Endosurgery, and USGI Medical – Grant/Research Support. Endogenex technology licensed by Mayo Clinic, with institutional equity and royalty through Mayo Clinic's invention policy. – coinventor.
Ryan Law: Boston Scientific – Consultant, Grant/Research Support. Neptune Medical – Data safety monitoring board. Olympus America – Consultant, Grant/Research Support. UpToDate – Royalties.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Rishad Khan, MD1, Barham Abu Dayyeh, MD, MPH2, Ryan Law, DO1, Andrew Storm, MD1. P1463 - Use of a Novel Electrosurgical Cystotome for Biliary Stricture Dilation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
