Sunday Poster Session
Category: Interventional Endoscopy
P1456 - Real-World Use of EUS-Guided Ethanol Ablation for Small pNETs: Patient-Centered Alternatives to Surgery
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Adedamola Bello, MD (he/him/his)
University of Saskatchewan
Saskatoon, SK, Canada
Presenting Author(s)
Adedamola Bello, MD1, Roberto Trasolini, MD, MSc2, Sidhartha Gupta, MBBS2
1University of Saskatchewan, Saskatoon, SK, Canada; 2University of British Columbia, Vancouver General Hospital, Vancouver, BC, Canada
Introduction: Pancreatic neuroendocrine tumors (pNETs) are increasingly diagnosed incidentally, with small, non-functional lesions often presenting a management dilemma. While surgical resection remains the standard of care for localized disease, it carries significant morbidity, particularly for lesions in the pancreatic head. Endoscopic ultrasound-guided ethanol ablation (EUS-EA) has emerged as a minimally invasive, pancreas-sparing alternative for select patients, including those who are medically inoperable, prefer organ-preserving therapy, or have hereditary tumor predisposition syndromes such as multiple endocrine neoplasia type 1 (MEN1). Despite growing international experience, real-world data on EUS-EA in Canadian practice remain limited.
Case Description/
Methods: We retrospectively reviewed three cases (ages 34–87) of biopsy-confirmed WHO Grade 1 pNETs measuring ≤1.5 cm, including one with MEN1 syndrome. All patients underwent EUS-guided ethanol ablation using 0.5–1.2 mL of 100% ethanol under conscious sedation. Procedures were performed by interventional endoscopists. Follow-up imaging at 3–6 months assessed treatment response, and patients were monitored clinically for complications and symptom recurrence.
Discussion: All three patients underwent technically successful EUS-EA without procedure-related adverse events. Two achieved complete radiographic response after a single session. One elderly patient with recurrent insulinoma required a second ablation for residual disease, with resolution of hypoglycemia without recurrence. Functional outcomes were favorable across all cases. One patient had confirmed MEN1; genetic counseling and endocrine follow-up were arranged. No pancreatitis or bleeding occurred.
This case series demonstrates the feasibility, efficacy and safety of EUS-guided ethanol ablation performed by interventional endoscopists for small, low-grade pNETs in a Canadian tertiary care setting. These findings support EUS-EA as a surgery-sparing option for carefully selected patients, particularly those unfit for resection or seeking minimally invasive alternatives. Our results align with emerging data on the use of ablative therapies in pNETs, demonstrating high technical success and favorable functional outcomes. Ongoing surveillance and larger prospective studies are warranted to define long-term durability and recurrence rates.
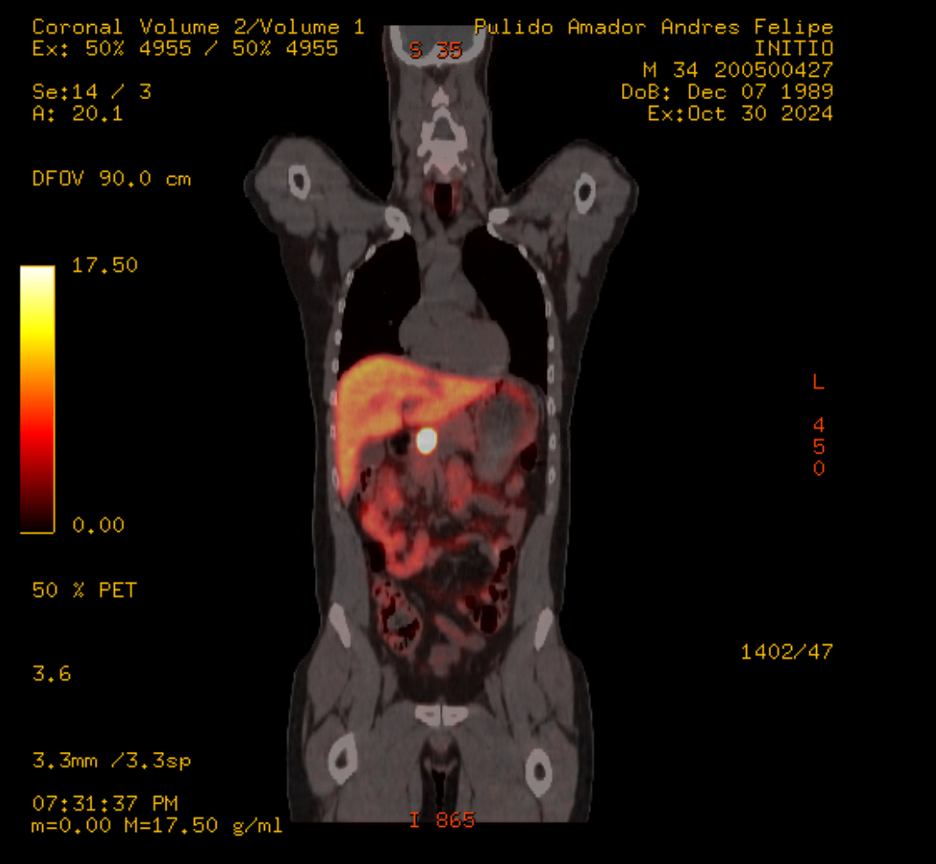
Figure: Figure 1: EUS image demonstrating ethanol injection into a 1.4 cm biopsy-confirmed insulinoma located in the pancreatic head. A 25G FNA needle is visualized traversing the duodenal wall and entering the lesion, which was treated with 1.2 mL of 100% ethanol under real-time guidance
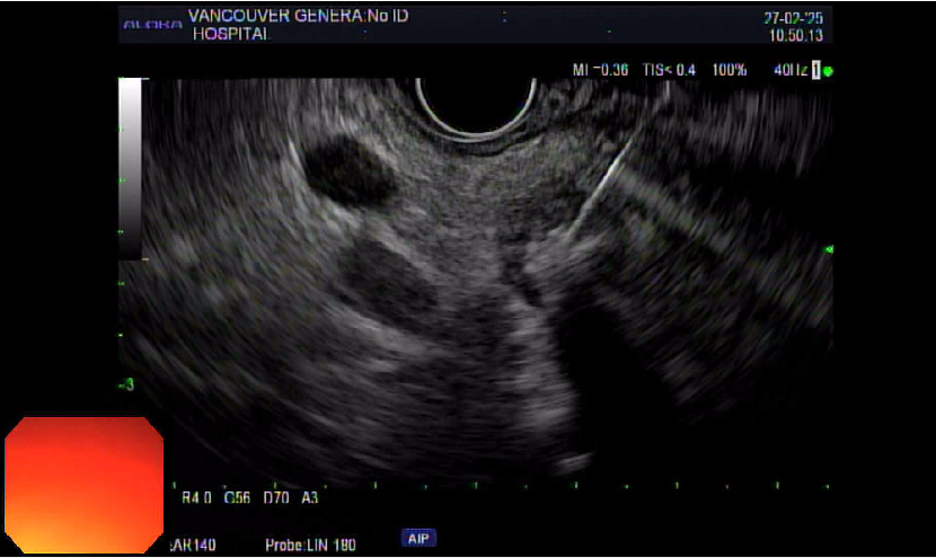
Figure: Figure 1B: DOTATE PET: 2.4 x 2.2 cm intensely tracer avid lesion (SUVmax 131.4) is seen within the pancreatic neck
Disclosures:
Adedamola Bello indicated no relevant financial relationships.
Roberto Trasolini indicated no relevant financial relationships.
Sidhartha Gupta indicated no relevant financial relationships.
Adedamola Bello, MD1, Roberto Trasolini, MD, MSc2, Sidhartha Gupta, MBBS2. P1456 - Real-World Use of EUS-Guided Ethanol Ablation for Small pNETs: Patient-Centered Alternatives to Surgery, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Saskatchewan, Saskatoon, SK, Canada; 2University of British Columbia, Vancouver General Hospital, Vancouver, BC, Canada
Introduction: Pancreatic neuroendocrine tumors (pNETs) are increasingly diagnosed incidentally, with small, non-functional lesions often presenting a management dilemma. While surgical resection remains the standard of care for localized disease, it carries significant morbidity, particularly for lesions in the pancreatic head. Endoscopic ultrasound-guided ethanol ablation (EUS-EA) has emerged as a minimally invasive, pancreas-sparing alternative for select patients, including those who are medically inoperable, prefer organ-preserving therapy, or have hereditary tumor predisposition syndromes such as multiple endocrine neoplasia type 1 (MEN1). Despite growing international experience, real-world data on EUS-EA in Canadian practice remain limited.
Case Description/
Methods: We retrospectively reviewed three cases (ages 34–87) of biopsy-confirmed WHO Grade 1 pNETs measuring ≤1.5 cm, including one with MEN1 syndrome. All patients underwent EUS-guided ethanol ablation using 0.5–1.2 mL of 100% ethanol under conscious sedation. Procedures were performed by interventional endoscopists. Follow-up imaging at 3–6 months assessed treatment response, and patients were monitored clinically for complications and symptom recurrence.
Discussion: All three patients underwent technically successful EUS-EA without procedure-related adverse events. Two achieved complete radiographic response after a single session. One elderly patient with recurrent insulinoma required a second ablation for residual disease, with resolution of hypoglycemia without recurrence. Functional outcomes were favorable across all cases. One patient had confirmed MEN1; genetic counseling and endocrine follow-up were arranged. No pancreatitis or bleeding occurred.
This case series demonstrates the feasibility, efficacy and safety of EUS-guided ethanol ablation performed by interventional endoscopists for small, low-grade pNETs in a Canadian tertiary care setting. These findings support EUS-EA as a surgery-sparing option for carefully selected patients, particularly those unfit for resection or seeking minimally invasive alternatives. Our results align with emerging data on the use of ablative therapies in pNETs, demonstrating high technical success and favorable functional outcomes. Ongoing surveillance and larger prospective studies are warranted to define long-term durability and recurrence rates.

Figure: Figure 1: EUS image demonstrating ethanol injection into a 1.4 cm biopsy-confirmed insulinoma located in the pancreatic head. A 25G FNA needle is visualized traversing the duodenal wall and entering the lesion, which was treated with 1.2 mL of 100% ethanol under real-time guidance

Figure: Figure 1B: DOTATE PET: 2.4 x 2.2 cm intensely tracer avid lesion (SUVmax 131.4) is seen within the pancreatic neck
Disclosures:
Adedamola Bello indicated no relevant financial relationships.
Roberto Trasolini indicated no relevant financial relationships.
Sidhartha Gupta indicated no relevant financial relationships.
Adedamola Bello, MD1, Roberto Trasolini, MD, MSc2, Sidhartha Gupta, MBBS2. P1456 - Real-World Use of EUS-Guided Ethanol Ablation for Small pNETs: Patient-Centered Alternatives to Surgery, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

