Sunday Poster Session
Category: Liver
P1556 - Association Between Immune Checkpoint Inhibitor Therapy and De Novo Autoimmune Hepatitis Development in Cancer Patients
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Danah Al-Deiri, MD (she/her/hers)
Cleveland Clinic Foundation
Cleveland, OH
Presenting Author(s)
Danah Al-Deiri, MD1, Sara Haddad, MD2, Julia Wajsberg, MD3, Joseph Sleiman, MD1, Shilpa Junna, MD1, Jessica R. Philpott, MD, PhD, FACG1
1Cleveland Clinic Foundation, Cleveland, OH; 2Cleveland Clinic, Cleveland, OH; 3University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH
Introduction: Immune checkpoint inhibitors are known to cause hepatotoxicity as an immune-related adverse event. However, the risk of de novo autoimmune hepatitis (AIH) following ICI therapy has not been well established. This study aimed to evaluate the incidence of new AIH coding in cancer patients receiving ICIs compared to those receiving conventional (non-ICI) cancer therapies.
Methods: We conducted a retrospective cohort study using the TriNetX real-world database from March 25, 2011 to January 1, 2025. Adult cancer patients (age >18 years) with elevated liver enzymes occurring 6 months after treatment initiation were included across all cancer types. Patients with pre-existing AIH were excluded. he ICI-exposed group (recipients of PD-1, PD-L1, or CTLA-4 inhibitors) was propensity score-matched 1:1 to non-ICI-exposed patients based on age, sex, demographics, and cancer type. The primary outcome was new AIH coding within one year of therapy initiation. Statistical significance was defined as p< 0.05.
Results: After propensity matching, 2,092 patients were included in each group. The mean age was 64.9 years in both groups, with 49% and 50.3% female patients in the exposed and non-exposed groups, respectively (Table 1). De novo AIH was coded in 0.6% (13/2,092) of ICI-treated patients compared to 0% (0/2,092) of non-exposed patients. The risk difference was 0.6% (95% CI: 0.32%, 1.019%; p = 0.0002), representing a statistically significant increased risk of de novo AIH codingin patients receiving ICIs.
Discussion: ICI therapy was associated with a significantly higher risk of new autoimmune hepatitis coding compared to non-ICI cancer treatment. While rare, this potential adverse effect should be considered in the differential diagnosis of liver enzyme elevation in ICI recipients. Further studies are warranted to clinically validate these findings, assess diagnostic accuracy, and evaluate long-term outcomes of ICI-associated autoimmune hepatitis.
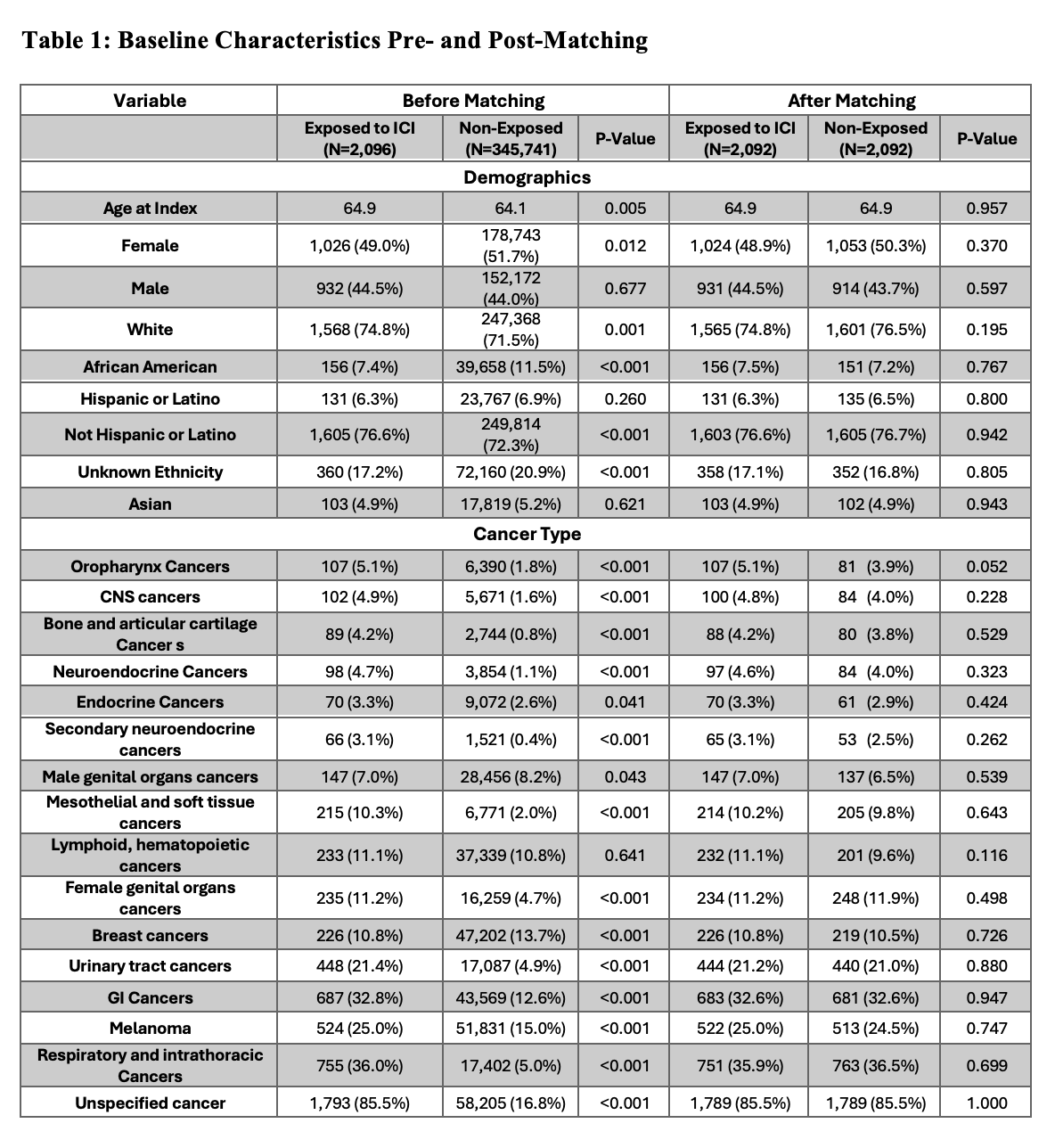
Figure: Comparison of baseline demographics and cancer diagnoses between study groups before and after propensity score matching
Disclosures:
Danah Al-Deiri indicated no relevant financial relationships.
Sara Haddad indicated no relevant financial relationships.
Julia Wajsberg indicated no relevant financial relationships.
Joseph Sleiman indicated no relevant financial relationships.
Shilpa Junna indicated no relevant financial relationships.
Jessica Philpott: Abbvie – Speakers Bureau.
Danah Al-Deiri, MD1, Sara Haddad, MD2, Julia Wajsberg, MD3, Joseph Sleiman, MD1, Shilpa Junna, MD1, Jessica R. Philpott, MD, PhD, FACG1. P1556 - Association Between Immune Checkpoint Inhibitor Therapy and De Novo Autoimmune Hepatitis Development in Cancer Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cleveland Clinic Foundation, Cleveland, OH; 2Cleveland Clinic, Cleveland, OH; 3University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH
Introduction: Immune checkpoint inhibitors are known to cause hepatotoxicity as an immune-related adverse event. However, the risk of de novo autoimmune hepatitis (AIH) following ICI therapy has not been well established. This study aimed to evaluate the incidence of new AIH coding in cancer patients receiving ICIs compared to those receiving conventional (non-ICI) cancer therapies.
Methods: We conducted a retrospective cohort study using the TriNetX real-world database from March 25, 2011 to January 1, 2025. Adult cancer patients (age >18 years) with elevated liver enzymes occurring 6 months after treatment initiation were included across all cancer types. Patients with pre-existing AIH were excluded. he ICI-exposed group (recipients of PD-1, PD-L1, or CTLA-4 inhibitors) was propensity score-matched 1:1 to non-ICI-exposed patients based on age, sex, demographics, and cancer type. The primary outcome was new AIH coding within one year of therapy initiation. Statistical significance was defined as p< 0.05.
Results: After propensity matching, 2,092 patients were included in each group. The mean age was 64.9 years in both groups, with 49% and 50.3% female patients in the exposed and non-exposed groups, respectively (Table 1). De novo AIH was coded in 0.6% (13/2,092) of ICI-treated patients compared to 0% (0/2,092) of non-exposed patients. The risk difference was 0.6% (95% CI: 0.32%, 1.019%; p = 0.0002), representing a statistically significant increased risk of de novo AIH codingin patients receiving ICIs.
Discussion: ICI therapy was associated with a significantly higher risk of new autoimmune hepatitis coding compared to non-ICI cancer treatment. While rare, this potential adverse effect should be considered in the differential diagnosis of liver enzyme elevation in ICI recipients. Further studies are warranted to clinically validate these findings, assess diagnostic accuracy, and evaluate long-term outcomes of ICI-associated autoimmune hepatitis.

Figure: Comparison of baseline demographics and cancer diagnoses between study groups before and after propensity score matching
Disclosures:
Danah Al-Deiri indicated no relevant financial relationships.
Sara Haddad indicated no relevant financial relationships.
Julia Wajsberg indicated no relevant financial relationships.
Joseph Sleiman indicated no relevant financial relationships.
Shilpa Junna indicated no relevant financial relationships.
Jessica Philpott: Abbvie – Speakers Bureau.
Danah Al-Deiri, MD1, Sara Haddad, MD2, Julia Wajsberg, MD3, Joseph Sleiman, MD1, Shilpa Junna, MD1, Jessica R. Philpott, MD, PhD, FACG1. P1556 - Association Between Immune Checkpoint Inhibitor Therapy and De Novo Autoimmune Hepatitis Development in Cancer Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
