Sunday Poster Session
Category: Liver
P1646 - BMI Does Not Differentiate MASLD From Metabolic Controls in Non-Obese Individuals
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- VP
Vinathi Polamraju, MD
Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital
St. Louis, MO
Presenting Author(s)
Vinathi Polamraju, MD1, Kamilah Scales, MD2, Peter McDonnell, MD1, Scott McHenry, MD, MSc1, Cassandra D.L. Fritz, MD, MPHS3
1Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital, St. Louis, MO; 2Washington University School of Medicine in St. Louis, St. Louis, MO; 3Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital, Saint Louis, MO
Introduction: The diagnosis of metabolic dysfunction-associated steatotic liver disease (MASLD) requires the presence of metabolic comorbidities or elevated body mass index (BMI). However, in nonobese individuals (BMI < 30kg/m2), the clinical utility of BMI to distinguish patients with MASLD from metabolically similar individuals without imaging evidence of hepatic steatosis is not well-established.
Methods: This retrospective single-center study included 423 adult primary care patients with BMI < 30 kg/m² without significant alcohol use or secondary causes of liver disease. Patients were classified as having MASLD if they had hepatic steatosis plus ≥2 metabolic comorbidities or type 2 diabetes alone (n=103), and as metabolic controls if they had no steatosis but met the same metabolic criteria (n=320). Metabolic comorbidities were defined as hypertension, hyperlipidemia, and prediabetes. Median BMI and interquartile ranges (IQR) were compared across groups. Univariate and multivariable logistic regression analyses assessed associations with MASLD versus metabolic controls. The multivariable model adjusted for age, sex, race, BMI, hypertension, hyperlipidemia, and type 2 diabetes. Backward stepwise selection was applied to identify significant independent predictors.
Results: Median BMI was higher in MASLD (26.6 kg/m², IQR 5.26) compared to controls (25.1 kg/m², IQR 5.06; p = 0.008), but this difference did not persist in multivariate modeling. In univariate analysis, MASLD was associated with higher rates of hypertension (83% vs. 94%, p < 0.001), but BMI itself was not a significant predictor (p = 0.25). In multivariate regression, hyperlipidemia (aOR 5.42, 95% CI 3.15–9.80, p < 0.001) was associated with MASLD, while African American race was inversely associated (aOR 0.61, 95% CI 0.37–1.01, p = 0.018). Type 2 diabetes, hypertension, and Asian race each had aOR>1, suggesting a likely association with MASLD, but did not reach statistical significance. BMI was not retained as an independent differentiating variable (Figure 1).
Discussion: Among nonobese individuals, BMI does not reliably distinguish those with MASLD from metabolic controls. Additionally, this study identifies African American race to be protective against MASLD. These findings challenge the reliance on BMI as a discriminative marker in lean or nonobese populations and support the need for improved diagnostic strategies that incorporate metabolic and racial risk profiles independent of BMI.
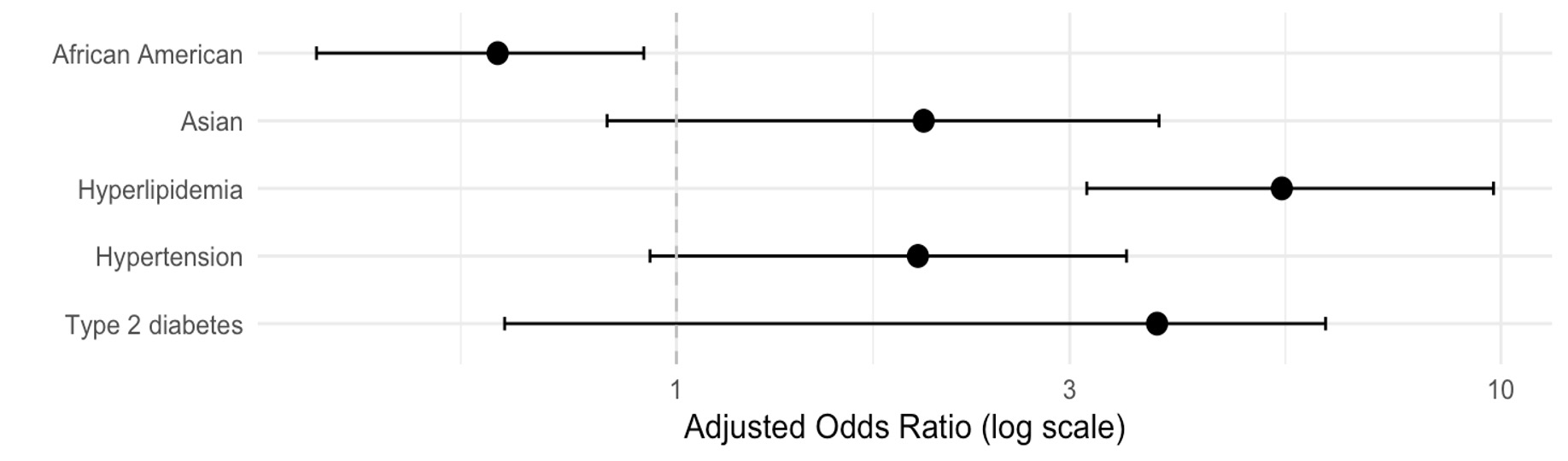
Figure: Independent Predictors of MASLD Among Non-Obese Patients Compared to At-Risk Metabolic Controls Without Hepatic Steatosis
*Multivariable logistic regression analysis adjusted for age, sex, race (White, African American, Asian), BMI, hypertension, hyperlipidemia, type 2 diabetes, and pre-diabetes
Disclosures:
Vinathi Polamraju indicated no relevant financial relationships.
Kamilah Scales indicated no relevant financial relationships.
Peter McDonnell indicated no relevant financial relationships.
Scott McHenry indicated no relevant financial relationships.
Cassandra Fritz indicated no relevant financial relationships.
Vinathi Polamraju, MD1, Kamilah Scales, MD2, Peter McDonnell, MD1, Scott McHenry, MD, MSc1, Cassandra D.L. Fritz, MD, MPHS3. P1646 - BMI Does Not Differentiate MASLD From Metabolic Controls in Non-Obese Individuals, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital, St. Louis, MO; 2Washington University School of Medicine in St. Louis, St. Louis, MO; 3Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital, Saint Louis, MO
Introduction: The diagnosis of metabolic dysfunction-associated steatotic liver disease (MASLD) requires the presence of metabolic comorbidities or elevated body mass index (BMI). However, in nonobese individuals (BMI < 30kg/m2), the clinical utility of BMI to distinguish patients with MASLD from metabolically similar individuals without imaging evidence of hepatic steatosis is not well-established.
Methods: This retrospective single-center study included 423 adult primary care patients with BMI < 30 kg/m² without significant alcohol use or secondary causes of liver disease. Patients were classified as having MASLD if they had hepatic steatosis plus ≥2 metabolic comorbidities or type 2 diabetes alone (n=103), and as metabolic controls if they had no steatosis but met the same metabolic criteria (n=320). Metabolic comorbidities were defined as hypertension, hyperlipidemia, and prediabetes. Median BMI and interquartile ranges (IQR) were compared across groups. Univariate and multivariable logistic regression analyses assessed associations with MASLD versus metabolic controls. The multivariable model adjusted for age, sex, race, BMI, hypertension, hyperlipidemia, and type 2 diabetes. Backward stepwise selection was applied to identify significant independent predictors.
Results: Median BMI was higher in MASLD (26.6 kg/m², IQR 5.26) compared to controls (25.1 kg/m², IQR 5.06; p = 0.008), but this difference did not persist in multivariate modeling. In univariate analysis, MASLD was associated with higher rates of hypertension (83% vs. 94%, p < 0.001), but BMI itself was not a significant predictor (p = 0.25). In multivariate regression, hyperlipidemia (aOR 5.42, 95% CI 3.15–9.80, p < 0.001) was associated with MASLD, while African American race was inversely associated (aOR 0.61, 95% CI 0.37–1.01, p = 0.018). Type 2 diabetes, hypertension, and Asian race each had aOR>1, suggesting a likely association with MASLD, but did not reach statistical significance. BMI was not retained as an independent differentiating variable (Figure 1).
Discussion: Among nonobese individuals, BMI does not reliably distinguish those with MASLD from metabolic controls. Additionally, this study identifies African American race to be protective against MASLD. These findings challenge the reliance on BMI as a discriminative marker in lean or nonobese populations and support the need for improved diagnostic strategies that incorporate metabolic and racial risk profiles independent of BMI.

Figure: Independent Predictors of MASLD Among Non-Obese Patients Compared to At-Risk Metabolic Controls Without Hepatic Steatosis
*Multivariable logistic regression analysis adjusted for age, sex, race (White, African American, Asian), BMI, hypertension, hyperlipidemia, type 2 diabetes, and pre-diabetes
Disclosures:
Vinathi Polamraju indicated no relevant financial relationships.
Kamilah Scales indicated no relevant financial relationships.
Peter McDonnell indicated no relevant financial relationships.
Scott McHenry indicated no relevant financial relationships.
Cassandra Fritz indicated no relevant financial relationships.
Vinathi Polamraju, MD1, Kamilah Scales, MD2, Peter McDonnell, MD1, Scott McHenry, MD, MSc1, Cassandra D.L. Fritz, MD, MPHS3. P1646 - BMI Does Not Differentiate MASLD From Metabolic Controls in Non-Obese Individuals, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
