Sunday Poster Session
Category: Liver
P1703 - Recurrent Hepatic Adenoma Post-Orthotopic Liver Transplant in Heterozygote Glycogen Storage Disease Type Ia: Implications for Genetic and Immune-Mediated Pathways
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Dayoung Jeon, MD
Houston Methodist Hospital
Houston, TX
Presenting Author(s)
Dayoung Jeon, MD1, Brandon Fong, BS2, Nandini Ray, MD1, Ahmad Afzal, MD1
1Houston Methodist Hospital, Houston, TX; 2Texas A&M School of Medicine, Houston, TX
Introduction: Glycogen storage disease type Ia (GSD-Ia), caused by G6PC mutations, predisposes patients to hepatic adenomas (HCA) in 70–80% of cases. Liver transplantation (LT) corrects metabolic derangements but carries a 22% risk of HCA recurrence, often linked to genetic drivers like β-catenin (CTNNB1) or HNF1A mutations. This case highlights recurrent HCA in a heterozygote GSD-Ia patient post-LT, underscoring unresolved genetic and immune mechanisms.
Case Description/
Methods: A 21-year-old male with heterozygous glycogen storage disease type I (GSD-I) underwent orthotopic liver transplant (OLT) in 2010 for multiple hepatic adenomas. He had no history of hepatitis, liver risk factors, or family history of hepatobiliary disease. Pre-transplant genetic testing was inconclusive for GSD but revealed APC and TP53 mutations, suggesting a genetic predisposition to neoplasia. Post-OLT, he experienced early rejection managed with prednisone, followed by alloimmune hepatitis requiring triple immunosuppression, which led to cognitive decline. Liver biopsy showed portal fibrosis and plasma cell-rich interface hepatitis.
Surveillance imaging in 2017 revealed five small, FNH-like liver lesions, stable on follow-up. By 2022, labs showed elevated bilirubin, AST, and ammonia, but imaging remained unchanged. In 2024, MRI identified a new 3 cm hepatic mass and two smaller lesions, confirmed as recurrent hepatic adenomas on biopsy. Repeat genetic testing confirmed heterozygous GSD-I. This case highlights the complexity of hepatic neoplasms in GSD-I post-OLT, suggesting that genetic predisposition and chronic inflammation may drive recurrence despite metabolic correction, underscoring the need for ongoing surveillance and genetic evaluation.
Discussion: Recurrent hepatocellular adenoma post-LT challenges the paradigm of metabolic cure in GSD-Ia. Heterozygosity may permit subclinical glycogen accumulation, while IST and chronic inflammation could drive CTNNB1 activation. The coexistence of APC and TP53 mutations suggests a polygenic predisposition, warranting the use of next-generation sequencing to clarify oncogenic pathways. This case highlights the importance of lifelong surveillance in GSD-Ia patients after liver transplantation (LT), even in the presence of metabolic stability. Genetic and immune-mediated mechanisms may synergize to promote HCA recurrence, necessitating research into targeted therapies and risk stratification.
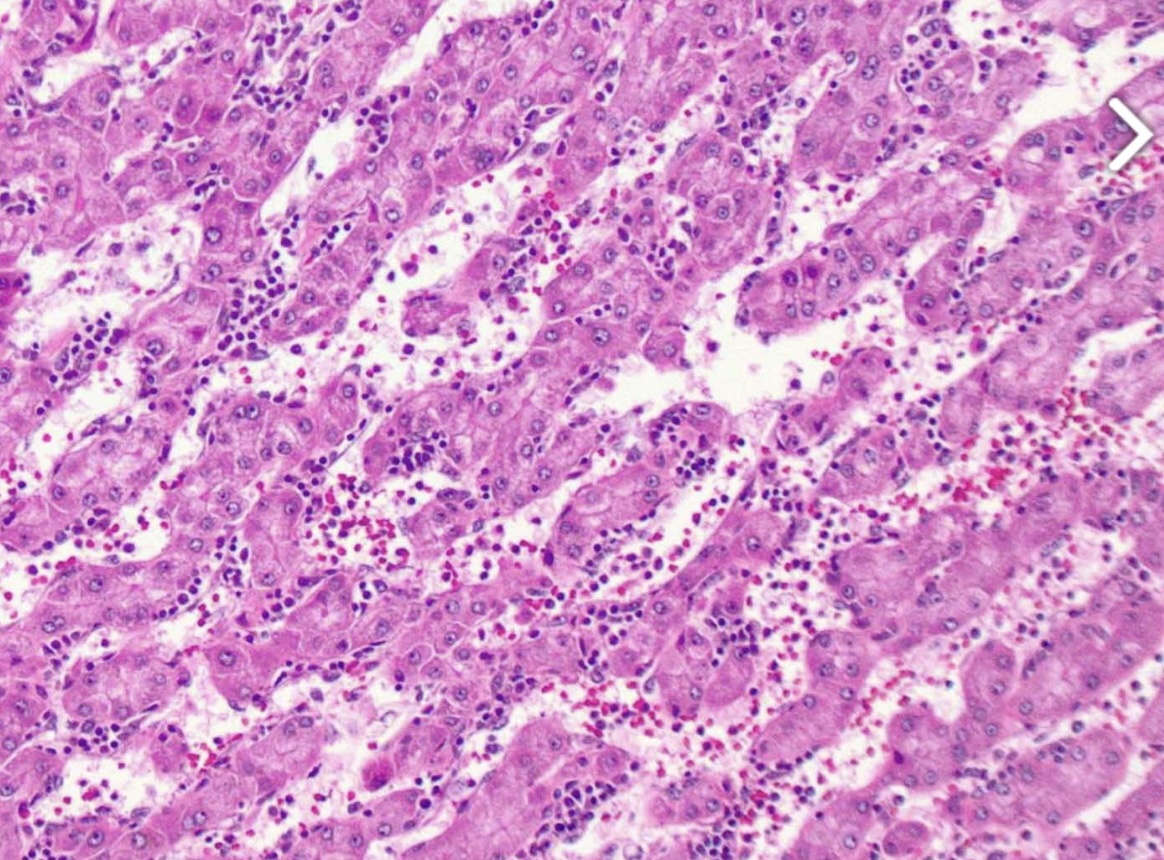
Figure: A histological image of the liver mass, which shows hepatocellular adenoma.
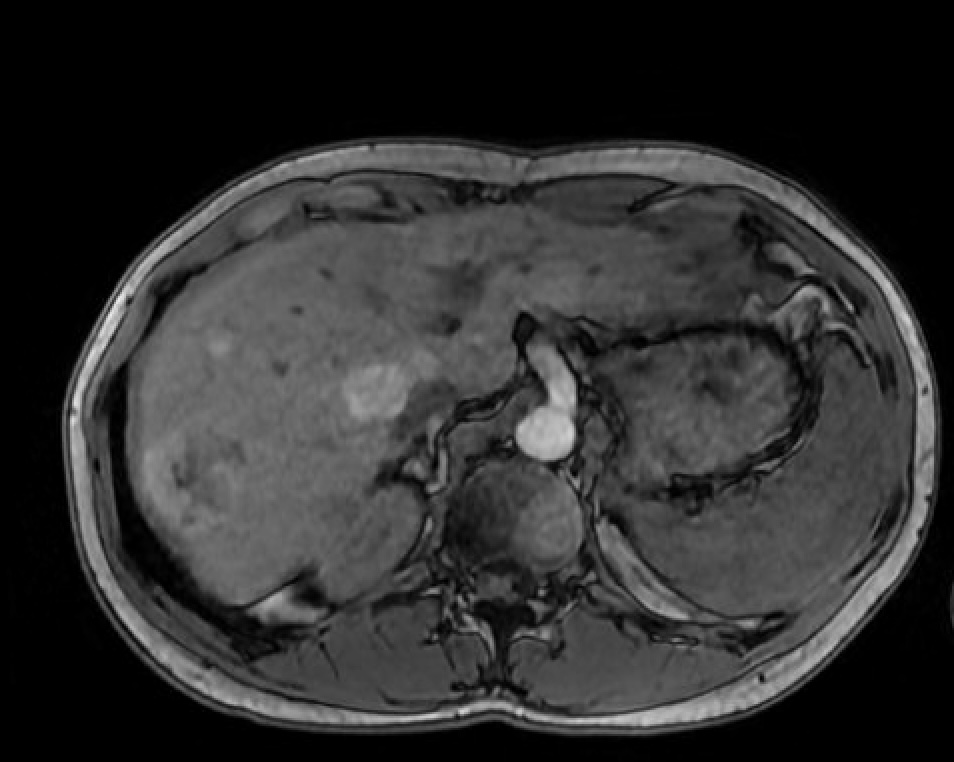
Figure: Axial image of MRI abdomen showing multifocal masses, largest mass measuring 2.98 cm x 2.95 cm
Disclosures:
Dayoung Jeon indicated no relevant financial relationships.
Brandon Fong indicated no relevant financial relationships.
Nandini Ray indicated no relevant financial relationships.
Ahmad Afzal indicated no relevant financial relationships.
Dayoung Jeon, MD1, Brandon Fong, BS2, Nandini Ray, MD1, Ahmad Afzal, MD1. P1703 - Recurrent Hepatic Adenoma Post-Orthotopic Liver Transplant in Heterozygote Glycogen Storage Disease Type Ia: Implications for Genetic and Immune-Mediated Pathways, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Houston Methodist Hospital, Houston, TX; 2Texas A&M School of Medicine, Houston, TX
Introduction: Glycogen storage disease type Ia (GSD-Ia), caused by G6PC mutations, predisposes patients to hepatic adenomas (HCA) in 70–80% of cases. Liver transplantation (LT) corrects metabolic derangements but carries a 22% risk of HCA recurrence, often linked to genetic drivers like β-catenin (CTNNB1) or HNF1A mutations. This case highlights recurrent HCA in a heterozygote GSD-Ia patient post-LT, underscoring unresolved genetic and immune mechanisms.
Case Description/
Methods: A 21-year-old male with heterozygous glycogen storage disease type I (GSD-I) underwent orthotopic liver transplant (OLT) in 2010 for multiple hepatic adenomas. He had no history of hepatitis, liver risk factors, or family history of hepatobiliary disease. Pre-transplant genetic testing was inconclusive for GSD but revealed APC and TP53 mutations, suggesting a genetic predisposition to neoplasia. Post-OLT, he experienced early rejection managed with prednisone, followed by alloimmune hepatitis requiring triple immunosuppression, which led to cognitive decline. Liver biopsy showed portal fibrosis and plasma cell-rich interface hepatitis.
Surveillance imaging in 2017 revealed five small, FNH-like liver lesions, stable on follow-up. By 2022, labs showed elevated bilirubin, AST, and ammonia, but imaging remained unchanged. In 2024, MRI identified a new 3 cm hepatic mass and two smaller lesions, confirmed as recurrent hepatic adenomas on biopsy. Repeat genetic testing confirmed heterozygous GSD-I. This case highlights the complexity of hepatic neoplasms in GSD-I post-OLT, suggesting that genetic predisposition and chronic inflammation may drive recurrence despite metabolic correction, underscoring the need for ongoing surveillance and genetic evaluation.
Discussion: Recurrent hepatocellular adenoma post-LT challenges the paradigm of metabolic cure in GSD-Ia. Heterozygosity may permit subclinical glycogen accumulation, while IST and chronic inflammation could drive CTNNB1 activation. The coexistence of APC and TP53 mutations suggests a polygenic predisposition, warranting the use of next-generation sequencing to clarify oncogenic pathways. This case highlights the importance of lifelong surveillance in GSD-Ia patients after liver transplantation (LT), even in the presence of metabolic stability. Genetic and immune-mediated mechanisms may synergize to promote HCA recurrence, necessitating research into targeted therapies and risk stratification.

Figure: A histological image of the liver mass, which shows hepatocellular adenoma.

Figure: Axial image of MRI abdomen showing multifocal masses, largest mass measuring 2.98 cm x 2.95 cm
Disclosures:
Dayoung Jeon indicated no relevant financial relationships.
Brandon Fong indicated no relevant financial relationships.
Nandini Ray indicated no relevant financial relationships.
Ahmad Afzal indicated no relevant financial relationships.
Dayoung Jeon, MD1, Brandon Fong, BS2, Nandini Ray, MD1, Ahmad Afzal, MD1. P1703 - Recurrent Hepatic Adenoma Post-Orthotopic Liver Transplant in Heterozygote Glycogen Storage Disease Type Ia: Implications for Genetic and Immune-Mediated Pathways, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
