Monday Poster Session
Category: Biliary/Pancreas
P2235 - Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis Prophylaxis; Does the Choice of Crystalloid Fluid Matter? A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- TA
Tareq Alsaleh, MD
Department of Internal Medicine, AdventHealth Orlando
Orlando, FL
Presenting Author(s)
Tareq Alsaleh, MD1, Nihal Khan, MD1, Abdul Mohammed, MD2, Mohamad Khaled Almujarkesh, MD2, Saurabh Chandan, MD3, Babu P. Mohan, MD4, John George, MD2, Maham Hayat, MD3, Deepanshu Jain, MD3, Natalie Cosgrove, MD3, Dennis Yang, MD, FACG5, Kambiz Kadkhodayan, MD3, Muhammad Hasan, MD, FACG3, Mustafa Arain, MD3
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL; 3Center for Interventional Endoscopy, AdventHealth Orlando, Orlando, FL; 4Orlando Gastroenterology PA, Orlando, FL; 5Center for Interventional Endoscopic, AdventHealth Orlando, Orlando, FL
Introduction: Intravenous (IV) hydration reduces the risk of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP), but the optimal crystalloid choice is not clear. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing IV hydration with lactate Ringer’s (LR) and normal saline (NS) for PEP prevention.
Methods: A systematic review of the literature from PubMed, Embase, Scopus, Web of Science, and Cochrane was conducted from inception to April 2025 to identify RCTs directly comparing LR and NS administered at equal rates during ERCP. The primary outcome of interest was the risk of post-ERCP pancreatitis. Secondary outcomes included other adverse events. Standard meta-analysis methods were followed using the random-effects model. Treatment effect estimates were expressed as risk difference (RD) and 95% confidence interval (CI). Heterogeneity was assessed using the I2% statistics.
Results: Three randomized controlled trials were included that reported on 619 patients undergoing ERCP. Males comprised 244 (39.4%) of those patients. Three hundred thirteen patients received LR, while 306 patients received NS. The LR group was 40.3% male, while the NS group was 38.6% male. Two trials were single-center, while one was a multi-center study. Rectal indomethacin was also used in two trials. Two trials included patients at high risk of PEP, while one included those at moderate-to-high risk (Table 1). High risk was defined per the European Society of Gastrointestinal Endoscopy (ESGE) guidelines as the presence of one major criterion or two minor criteria. Patients not meeting those criteria were classified as average risk.
There was a statistically significant risk reduction of PEP in the LR group compared to the NS group (RD -0.04; 95% CI [-0.08, -0.00], P=0.03, I2=0%). The two groups had no statistically significant risk difference in other reported adverse events, such as fluid overload, bleeding, or infection (RD -0.01; 95% CI [-0.04, 0.02], P=0.60, I2=0%). This data is summarized in Figure 1.
Discussion: Our meta-analysis found a statistically significant risk reduction of 4% with a number needed to treat (NNT) of 25 when using LR compared to NS at equivalent fluid administration rates for PEP prevention. LR may be the crystalloid of choice for patients undergoing ERCP, though further studies are needed to validate these results.
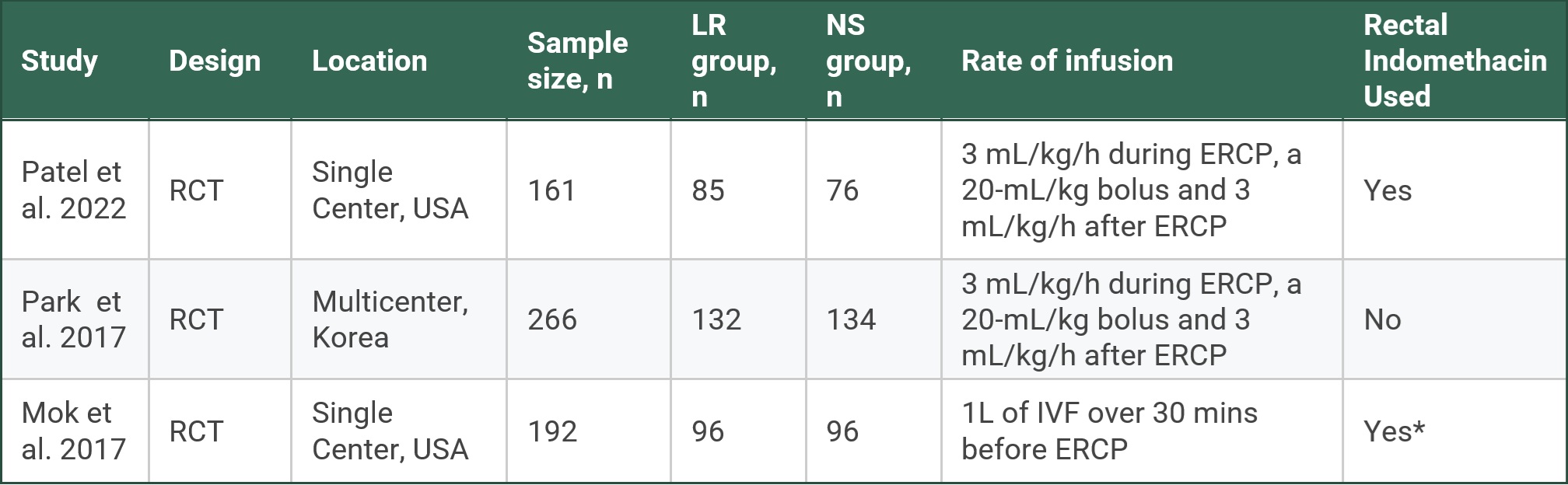
Figure: Table 1. Characteristics of individual studies included
*48 patients from each group, LR and NS, received rectal indomethacin while the other 48 patients did not

Figure: Figure 2. A – Pooled risk difference of post-ERCP pancreatitis in the LR group compared to the NS group. B – Pooled risk difference of other reported adverse events in the LR group compared to the NS group
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Nihal Khan indicated no relevant financial relationships.
Abdul Mohammed indicated no relevant financial relationships.
Mohamad Khaled Almujarkesh indicated no relevant financial relationships.
Saurabh Chandan indicated no relevant financial relationships.
Babu Mohan indicated no relevant financial relationships.
John George indicated no relevant financial relationships.
Maham Hayat indicated no relevant financial relationships.
Deepanshu Jain indicated no relevant financial relationships.
Natalie Cosgrove indicated no relevant financial relationships.
Dennis Yang: 3D-Matrix – Consultant. Apollo Endosurgery – Consultant. ERBE – Consultant. Fujifilm – Consultant. Medtronic – Consultant. MicroTech – Consultant. Olympus – Consultant.
Kambiz Kadkhodayan indicated no relevant financial relationships.
Muhammad Hasan: Boston Scientific – Consultant. MicroTech Endoscopy – Consultant. Olympus America – Consultant.
Mustafa Arain: Boston Scientific – Consultant. Cook Endoscopy – Consultant. Olympus – Consultant.
Tareq Alsaleh, MD1, Nihal Khan, MD1, Abdul Mohammed, MD2, Mohamad Khaled Almujarkesh, MD2, Saurabh Chandan, MD3, Babu P. Mohan, MD4, John George, MD2, Maham Hayat, MD3, Deepanshu Jain, MD3, Natalie Cosgrove, MD3, Dennis Yang, MD, FACG5, Kambiz Kadkhodayan, MD3, Muhammad Hasan, MD, FACG3, Mustafa Arain, MD3. P2235 - Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis Prophylaxis; Does the Choice of Crystalloid Fluid Matter? A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL; 3Center for Interventional Endoscopy, AdventHealth Orlando, Orlando, FL; 4Orlando Gastroenterology PA, Orlando, FL; 5Center for Interventional Endoscopic, AdventHealth Orlando, Orlando, FL
Introduction: Intravenous (IV) hydration reduces the risk of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP), but the optimal crystalloid choice is not clear. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing IV hydration with lactate Ringer’s (LR) and normal saline (NS) for PEP prevention.
Methods: A systematic review of the literature from PubMed, Embase, Scopus, Web of Science, and Cochrane was conducted from inception to April 2025 to identify RCTs directly comparing LR and NS administered at equal rates during ERCP. The primary outcome of interest was the risk of post-ERCP pancreatitis. Secondary outcomes included other adverse events. Standard meta-analysis methods were followed using the random-effects model. Treatment effect estimates were expressed as risk difference (RD) and 95% confidence interval (CI). Heterogeneity was assessed using the I2% statistics.
Results: Three randomized controlled trials were included that reported on 619 patients undergoing ERCP. Males comprised 244 (39.4%) of those patients. Three hundred thirteen patients received LR, while 306 patients received NS. The LR group was 40.3% male, while the NS group was 38.6% male. Two trials were single-center, while one was a multi-center study. Rectal indomethacin was also used in two trials. Two trials included patients at high risk of PEP, while one included those at moderate-to-high risk (Table 1). High risk was defined per the European Society of Gastrointestinal Endoscopy (ESGE) guidelines as the presence of one major criterion or two minor criteria. Patients not meeting those criteria were classified as average risk.
There was a statistically significant risk reduction of PEP in the LR group compared to the NS group (RD -0.04; 95% CI [-0.08, -0.00], P=0.03, I2=0%). The two groups had no statistically significant risk difference in other reported adverse events, such as fluid overload, bleeding, or infection (RD -0.01; 95% CI [-0.04, 0.02], P=0.60, I2=0%). This data is summarized in Figure 1.
Discussion: Our meta-analysis found a statistically significant risk reduction of 4% with a number needed to treat (NNT) of 25 when using LR compared to NS at equivalent fluid administration rates for PEP prevention. LR may be the crystalloid of choice for patients undergoing ERCP, though further studies are needed to validate these results.

Figure: Table 1. Characteristics of individual studies included
*48 patients from each group, LR and NS, received rectal indomethacin while the other 48 patients did not

Figure: Figure 2. A – Pooled risk difference of post-ERCP pancreatitis in the LR group compared to the NS group. B – Pooled risk difference of other reported adverse events in the LR group compared to the NS group
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Nihal Khan indicated no relevant financial relationships.
Abdul Mohammed indicated no relevant financial relationships.
Mohamad Khaled Almujarkesh indicated no relevant financial relationships.
Saurabh Chandan indicated no relevant financial relationships.
Babu Mohan indicated no relevant financial relationships.
John George indicated no relevant financial relationships.
Maham Hayat indicated no relevant financial relationships.
Deepanshu Jain indicated no relevant financial relationships.
Natalie Cosgrove indicated no relevant financial relationships.
Dennis Yang: 3D-Matrix – Consultant. Apollo Endosurgery – Consultant. ERBE – Consultant. Fujifilm – Consultant. Medtronic – Consultant. MicroTech – Consultant. Olympus – Consultant.
Kambiz Kadkhodayan indicated no relevant financial relationships.
Muhammad Hasan: Boston Scientific – Consultant. MicroTech Endoscopy – Consultant. Olympus America – Consultant.
Mustafa Arain: Boston Scientific – Consultant. Cook Endoscopy – Consultant. Olympus – Consultant.
Tareq Alsaleh, MD1, Nihal Khan, MD1, Abdul Mohammed, MD2, Mohamad Khaled Almujarkesh, MD2, Saurabh Chandan, MD3, Babu P. Mohan, MD4, John George, MD2, Maham Hayat, MD3, Deepanshu Jain, MD3, Natalie Cosgrove, MD3, Dennis Yang, MD, FACG5, Kambiz Kadkhodayan, MD3, Muhammad Hasan, MD, FACG3, Mustafa Arain, MD3. P2235 - Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis Prophylaxis; Does the Choice of Crystalloid Fluid Matter? A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
