Monday Poster Session
Category: Biliary/Pancreas
P2200 - The Economic Burden of Pancreatic Enzyme Replacement Therapy on Patients With Exocrine Pancreatic Insufficiency
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Kaitlin R. McGowan, DO (she/her/hers)
Dartmouth Hitchcock Medical Center
Lebanon, NH
Presenting Author(s)
Kaitlin R. McGowan, DO, Timothy B. Gardner, MD, MS, Gina N. Manzi, PharmD, Kerrington D. Smith, MD, Stuart R. Gordon, MD, Jeffrey M. Adler, MD
Dartmouth Hitchcock Medical Center, Lebanon, NH
Introduction: Pancreatic enzyme replacement therapy (PERT) are medications used to treat exocrine pancreatic exocrine insufficiency (EPI) that were originally excluded from the standard FDA pre-marketing approval process resulting in low cost due to many competing formulations being available. However, in 2009 the FDA mandated that companies marketing PERT had to undergo formal FDA approval and consequently the number of available PERT products dramatically decreased with an associated massive increase in cost. As of 2025, only 5 marketed PERT products still exist. Given their expense, we sought to evaluate the cost burden of PERT on patients with EPI.
Methods: A survey was given to patients presenting as outpatients to the gastroenterology and surgery clinics at a tertiary medical center in 2024-5 with a robust pancreatic diseases program. Surveys investigated patient demographics, reasons for taking PERT therapy, insurance type, and copay amount. Questions on affordability, if patients had noticed cost increases, and if patients had been exposed to marketing for PERT products were also included.
Results: 45 surveys were collected from 21 male and 24 female patients. The most common reasons for taking PEPs were pancreatectomy, followed by chronic pancreatitis and pancreatic cancer. All patients reported taking one of two different PERT products. Eleven patients reported paying >$300 monthly copay and an additional 3 patients reported paying $201-225 monthly for PERT therapy (Figure 1). Fifty-three percent of patients said PERT was difficult to afford, and 36% said they had missed doses due to cost. Thirty-six percent believed the cost of their PERT had increased from when they started therapy, and 44% were unsure. Eighty percent had not seen marketing or advertising by pharmaceutical companies for PERT in the past year.
Discussion: In 2025, the cost burden of vital PERT remains very high for over half of patients and is a significant barrier to patient compliance.
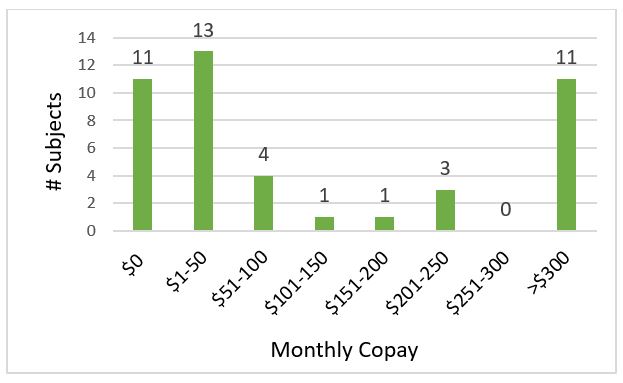
Figure: Figure 1. Monthly copay cost for pancreatic enzyme replacement therapy. Fourteen patients (31%) reported paying above $200 monthly for their prescriptions.
Disclosures:
Kaitlin McGowan indicated no relevant financial relationships.
Timothy Gardner indicated no relevant financial relationships.
Gina Manzi: Bristol Myers Squibb – Advisory Committee/Board Member. Janssen Pharmaceuticals – Advisory Committee/Board Member.
Kerrington Smith indicated no relevant financial relationships.
Stuart Gordon: Boston Scientific – Consultant.
Jeffrey Adler indicated no relevant financial relationships.
Kaitlin R. McGowan, DO, Timothy B. Gardner, MD, MS, Gina N. Manzi, PharmD, Kerrington D. Smith, MD, Stuart R. Gordon, MD, Jeffrey M. Adler, MD. P2200 - The Economic Burden of Pancreatic Enzyme Replacement Therapy on Patients With Exocrine Pancreatic Insufficiency, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Dartmouth Hitchcock Medical Center, Lebanon, NH
Introduction: Pancreatic enzyme replacement therapy (PERT) are medications used to treat exocrine pancreatic exocrine insufficiency (EPI) that were originally excluded from the standard FDA pre-marketing approval process resulting in low cost due to many competing formulations being available. However, in 2009 the FDA mandated that companies marketing PERT had to undergo formal FDA approval and consequently the number of available PERT products dramatically decreased with an associated massive increase in cost. As of 2025, only 5 marketed PERT products still exist. Given their expense, we sought to evaluate the cost burden of PERT on patients with EPI.
Methods: A survey was given to patients presenting as outpatients to the gastroenterology and surgery clinics at a tertiary medical center in 2024-5 with a robust pancreatic diseases program. Surveys investigated patient demographics, reasons for taking PERT therapy, insurance type, and copay amount. Questions on affordability, if patients had noticed cost increases, and if patients had been exposed to marketing for PERT products were also included.
Results: 45 surveys were collected from 21 male and 24 female patients. The most common reasons for taking PEPs were pancreatectomy, followed by chronic pancreatitis and pancreatic cancer. All patients reported taking one of two different PERT products. Eleven patients reported paying >$300 monthly copay and an additional 3 patients reported paying $201-225 monthly for PERT therapy (Figure 1). Fifty-three percent of patients said PERT was difficult to afford, and 36% said they had missed doses due to cost. Thirty-six percent believed the cost of their PERT had increased from when they started therapy, and 44% were unsure. Eighty percent had not seen marketing or advertising by pharmaceutical companies for PERT in the past year.
Discussion: In 2025, the cost burden of vital PERT remains very high for over half of patients and is a significant barrier to patient compliance.

Figure: Figure 1. Monthly copay cost for pancreatic enzyme replacement therapy. Fourteen patients (31%) reported paying above $200 monthly for their prescriptions.
Disclosures:
Kaitlin McGowan indicated no relevant financial relationships.
Timothy Gardner indicated no relevant financial relationships.
Gina Manzi: Bristol Myers Squibb – Advisory Committee/Board Member. Janssen Pharmaceuticals – Advisory Committee/Board Member.
Kerrington Smith indicated no relevant financial relationships.
Stuart Gordon: Boston Scientific – Consultant.
Jeffrey Adler indicated no relevant financial relationships.
Kaitlin R. McGowan, DO, Timothy B. Gardner, MD, MS, Gina N. Manzi, PharmD, Kerrington D. Smith, MD, Stuart R. Gordon, MD, Jeffrey M. Adler, MD. P2200 - The Economic Burden of Pancreatic Enzyme Replacement Therapy on Patients With Exocrine Pancreatic Insufficiency, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
