Monday Poster Session
Category: Esophagus
P2732 - Dupilumab Treatment Patterns for Eosinophilic Esophagitis in the United States: A Real-World Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- KP
Kathryn Peterson, MD
Division of Gastroenterology, University of Utah School of Medicine
Salt Lake City, UT
Presenting Author(s)
Kathryn A. Peterson, MD1, Rohan C. Parikh, PhD2, Fareedat Bello, MS2, Nami Pandit-Abid, PhD3, Bram P. Raphael, MD4, Alia Karaouni, PhD5, Ryan B. Thomas, PharmD, MS6, Sarette T. Tilton, PharmD3
1Division of Gastroenterology, University of Utah School of Medicine, Salt Lake City, UT; 2RTI Health Solutions, Research Triangle Park, NC; 3Sanofi, Cambridge, MA; 4Regeneron Pharmaceuticals Inc., Tarrytown, NY; 5BMAPS SARL, Geneva, Geneve, Switzerland; 6Regeneron Pharmaceuticals, Inc., Sleepy Hollow, NY
Introduction: Dupilumab, a fully human monoclonal antibody, inhibits interleukin (IL)-4 and IL-13 signaling pathways, which are central drivers of type 2 (T2) inflammation in eosinophilic esophagitis (EoE) and related T2 conditions. Clinical trials have established the efficacy and safety of dupilumab in children, adolescents and adults for the treatment of EoE. This study describes clinical characteristics and treatment patterns of EoE patients receiving dupilumab in routine clinical practice in the US.
Methods: In this retrospective study, records were extracted for newly diagnosed EoE patients (≥12 years) between January 01, 2018, and August 31, 2023 (index range; index is the EoE diagnosis date), confirmed by esophageal biopsy demonstrating peak eosinophil count (PEC) ≥15 eosinophils/high-power field (eos/hpf) within 90 days of diagnosis and had ≥1 known eosinophil count in the 12 months after index. We reported patient characteristics and treatment patterns for the subgroup of patients who received dupilumab for EoE between May 20, 2022, and August 31, 2023 (post-approval in EoE). Patients were followed for ≥12 months after initiating dupilumab, unless lost to follow-up. Results were summarized descriptively.
Results: This analysis included 52 EoE patients (mean age: 30.1 [SD: 15.0] years; 71.2% male). Of 52 patients, 38.5% (n=20) received dupilumab alone or in combination with proton pump inhibitor/swallowed topical corticosteroid (PPI/STC) as first treatments after EoE diagnosis, with all continuing dupilumab-based therapy till the end of follow-up (median duration: 2.2 years). Other first treatments included PPI alone (30.7%, n=16), and PPI-STC (26.9%, n=14) (Figure). Common EoE-related symptoms at diagnosis were dysphagia (69.2%, n=36), chest/throat pain (38.5%, n=20), and food impaction (34.6%, n=18). Findings at initial diagnosis: mean PEC, 40.8 (SD: 49.5) eos/hpf; esophageal abnormalities, 78.9% (n=41). Common T2 and other comorbidities included allergic rhinitis (38.5%, n=20), asthma (28.9%, n=15), and gastroesophageal reflux disease (44.2%, n=23) (Table).
Discussion: EoE patients treated with dupilumab had substantial symptom burden at diagnosis and high rates of comorbidities. Over one-third of dupilumab use was as first-line treatment. Results warrant cautious interpretation due to the low sample size. Understanding patient characteristics of dupilumab users may aid physicians in making informed decisions, particularly on biologic initiation in EoE.
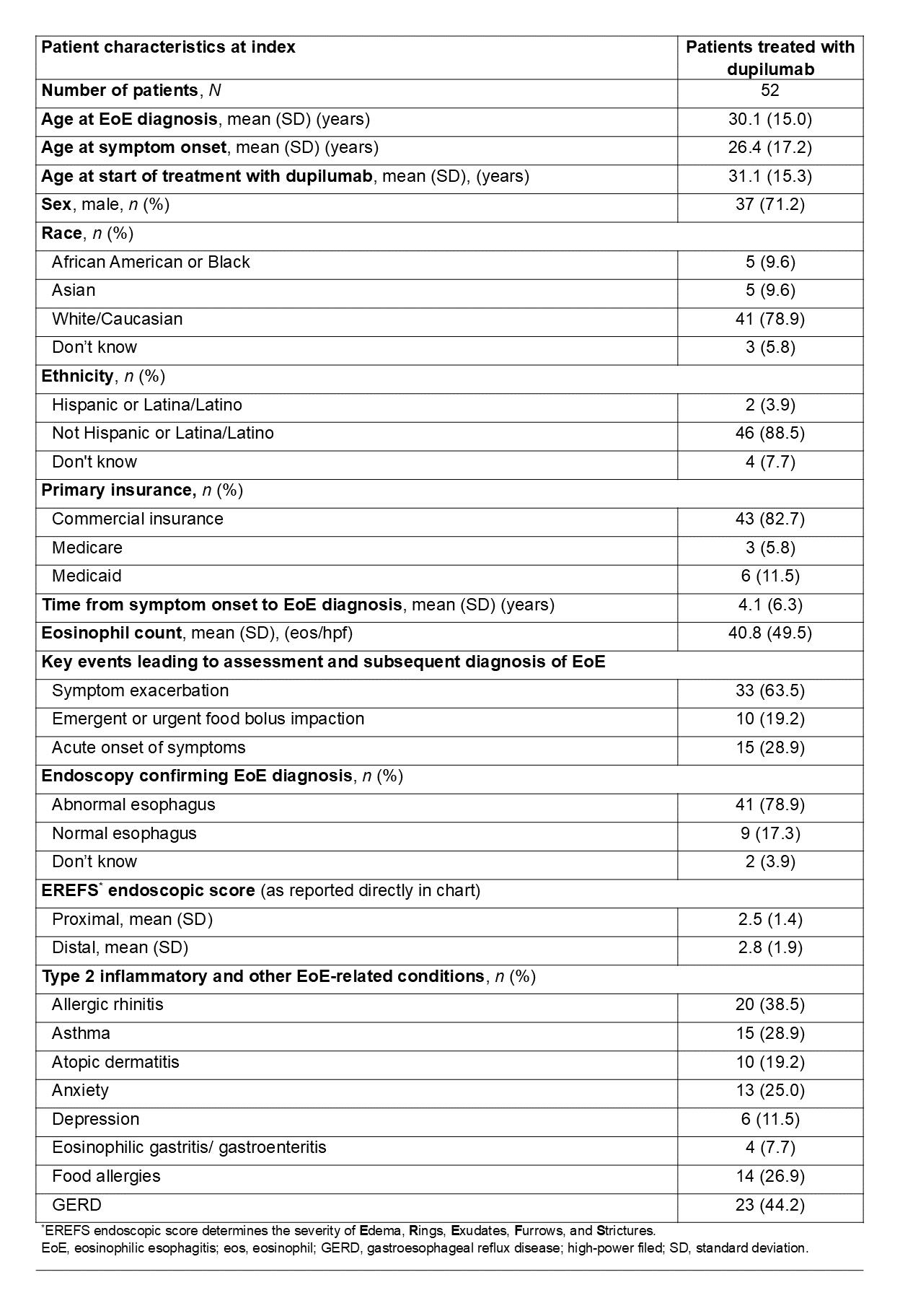
Figure: Figure. EoE treatment sequence in patients with EoE who initiated dupilumab, using a Sankey diagram
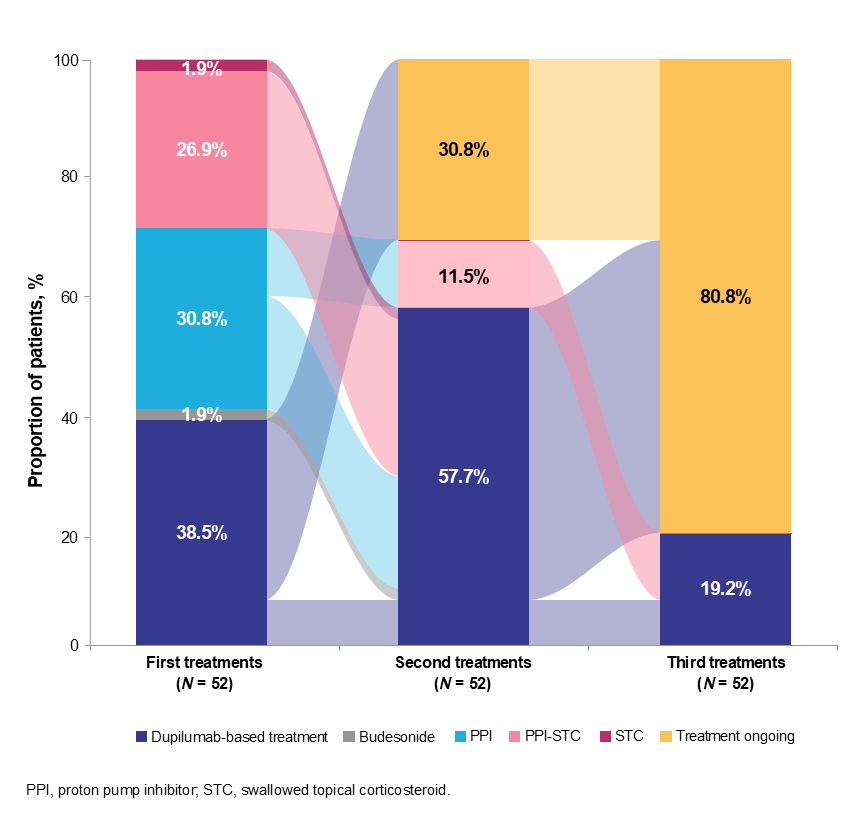
Figure: Table. Key baseline characteristics of EoE patients treated with dupilumab
Disclosures:
Kathryn A. Peterson: Adare – Grant/Research Support. AGA – Advisor or Review Panel Member, Consultant, Speakers Bureau. Alladapt – Advisor or Review Panel Member, Consultant. Allakos and Chobani – Grant/Research Support. AstraZeneca – Advisor or Review Panel Member, Consultant, Grant/Research Support. Bristol Meyers Squibb – Advisor or Review Panel Member, Consultant. Celldex – Grant/Research Support. Ellodi – Advisor or Review Panel Member, Consultant. Esocap – Advisor or Review Panel Member, Consultant. Invea – Advisor or Review Panel Member, Consultant. Lucid – Advisor or Review Panel Member, Consultant. Nexeos Bio – has equity. NIH – Grant/Research Support. Peerview – Advisor or Review Panel Member, Consultant, Speakers Bureau. Regeneron – Advisor or Review Panel Member, Consultant, Grant/Research Support. Revolo – Grant/Research Support. Sanofi – Grant/Research Support. Takeda – Advisor or Review Panel Member, Consultant, Speakers Bureau. Uniquity – Advisor or Review Panel Member, Consultant, Speakers Bureau. WebMD – Advisor or Review Panel Member, Consultant, Speakers Bureau.
Rohan C. Parikh: RTI Health Solutions – Employee. Sanofi – Grant/Research Support.
Fareedat Bello: RTI Health Solutions – Employee. Sanofi – Grant/Research Support.
Nami Pandit-Abid: Sanofi – Employee, Stock Options.
Bram Raphael: Regeneron Pharmaceuticals Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Alia Karaouni: BMAPS SARL – Employee. Sanofi – Consultant.
Ryan B. Thomas: Regeneron Pharmaceuticals, Inc – Employee, Stock Options.
Sarette T. Tilton: Sanofi – Employee, Stock Options.
Kathryn A. Peterson, MD1, Rohan C. Parikh, PhD2, Fareedat Bello, MS2, Nami Pandit-Abid, PhD3, Bram P. Raphael, MD4, Alia Karaouni, PhD5, Ryan B. Thomas, PharmD, MS6, Sarette T. Tilton, PharmD3. P2732 - Dupilumab Treatment Patterns for Eosinophilic Esophagitis in the United States: A Real-World Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Division of Gastroenterology, University of Utah School of Medicine, Salt Lake City, UT; 2RTI Health Solutions, Research Triangle Park, NC; 3Sanofi, Cambridge, MA; 4Regeneron Pharmaceuticals Inc., Tarrytown, NY; 5BMAPS SARL, Geneva, Geneve, Switzerland; 6Regeneron Pharmaceuticals, Inc., Sleepy Hollow, NY
Introduction: Dupilumab, a fully human monoclonal antibody, inhibits interleukin (IL)-4 and IL-13 signaling pathways, which are central drivers of type 2 (T2) inflammation in eosinophilic esophagitis (EoE) and related T2 conditions. Clinical trials have established the efficacy and safety of dupilumab in children, adolescents and adults for the treatment of EoE. This study describes clinical characteristics and treatment patterns of EoE patients receiving dupilumab in routine clinical practice in the US.
Methods: In this retrospective study, records were extracted for newly diagnosed EoE patients (≥12 years) between January 01, 2018, and August 31, 2023 (index range; index is the EoE diagnosis date), confirmed by esophageal biopsy demonstrating peak eosinophil count (PEC) ≥15 eosinophils/high-power field (eos/hpf) within 90 days of diagnosis and had ≥1 known eosinophil count in the 12 months after index. We reported patient characteristics and treatment patterns for the subgroup of patients who received dupilumab for EoE between May 20, 2022, and August 31, 2023 (post-approval in EoE). Patients were followed for ≥12 months after initiating dupilumab, unless lost to follow-up. Results were summarized descriptively.
Results: This analysis included 52 EoE patients (mean age: 30.1 [SD: 15.0] years; 71.2% male). Of 52 patients, 38.5% (n=20) received dupilumab alone or in combination with proton pump inhibitor/swallowed topical corticosteroid (PPI/STC) as first treatments after EoE diagnosis, with all continuing dupilumab-based therapy till the end of follow-up (median duration: 2.2 years). Other first treatments included PPI alone (30.7%, n=16), and PPI-STC (26.9%, n=14) (Figure). Common EoE-related symptoms at diagnosis were dysphagia (69.2%, n=36), chest/throat pain (38.5%, n=20), and food impaction (34.6%, n=18). Findings at initial diagnosis: mean PEC, 40.8 (SD: 49.5) eos/hpf; esophageal abnormalities, 78.9% (n=41). Common T2 and other comorbidities included allergic rhinitis (38.5%, n=20), asthma (28.9%, n=15), and gastroesophageal reflux disease (44.2%, n=23) (Table).
Discussion: EoE patients treated with dupilumab had substantial symptom burden at diagnosis and high rates of comorbidities. Over one-third of dupilumab use was as first-line treatment. Results warrant cautious interpretation due to the low sample size. Understanding patient characteristics of dupilumab users may aid physicians in making informed decisions, particularly on biologic initiation in EoE.

Figure: Figure. EoE treatment sequence in patients with EoE who initiated dupilumab, using a Sankey diagram

Figure: Table. Key baseline characteristics of EoE patients treated with dupilumab
Disclosures:
Kathryn A. Peterson: Adare – Grant/Research Support. AGA – Advisor or Review Panel Member, Consultant, Speakers Bureau. Alladapt – Advisor or Review Panel Member, Consultant. Allakos and Chobani – Grant/Research Support. AstraZeneca – Advisor or Review Panel Member, Consultant, Grant/Research Support. Bristol Meyers Squibb – Advisor or Review Panel Member, Consultant. Celldex – Grant/Research Support. Ellodi – Advisor or Review Panel Member, Consultant. Esocap – Advisor or Review Panel Member, Consultant. Invea – Advisor or Review Panel Member, Consultant. Lucid – Advisor or Review Panel Member, Consultant. Nexeos Bio – has equity. NIH – Grant/Research Support. Peerview – Advisor or Review Panel Member, Consultant, Speakers Bureau. Regeneron – Advisor or Review Panel Member, Consultant, Grant/Research Support. Revolo – Grant/Research Support. Sanofi – Grant/Research Support. Takeda – Advisor or Review Panel Member, Consultant, Speakers Bureau. Uniquity – Advisor or Review Panel Member, Consultant, Speakers Bureau. WebMD – Advisor or Review Panel Member, Consultant, Speakers Bureau.
Rohan C. Parikh: RTI Health Solutions – Employee. Sanofi – Grant/Research Support.
Fareedat Bello: RTI Health Solutions – Employee. Sanofi – Grant/Research Support.
Nami Pandit-Abid: Sanofi – Employee, Stock Options.
Bram Raphael: Regeneron Pharmaceuticals Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Alia Karaouni: BMAPS SARL – Employee. Sanofi – Consultant.
Ryan B. Thomas: Regeneron Pharmaceuticals, Inc – Employee, Stock Options.
Sarette T. Tilton: Sanofi – Employee, Stock Options.
Kathryn A. Peterson, MD1, Rohan C. Parikh, PhD2, Fareedat Bello, MS2, Nami Pandit-Abid, PhD3, Bram P. Raphael, MD4, Alia Karaouni, PhD5, Ryan B. Thomas, PharmD, MS6, Sarette T. Tilton, PharmD3. P2732 - Dupilumab Treatment Patterns for Eosinophilic Esophagitis in the United States: A Real-World Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
