Monday Poster Session
Category: Esophagus
P2775 - Baseline Characteristics of Patients With Reflux Esophagitis Receiving Vonoprazan Treatment in China: Post Hoc Analysis of the VIEW Real-World Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- FZ
Fang Zhou, MSc
Takeda Pharmaceutical Company, Shanghai, China
Shanghai, Shanghai, China
Presenting Author(s)
Yinglian Xiao, MD, PhD1, Kailun Liang, MSc2, Fang Zhou, MSc3, Minhu Chen, MD, PhD1
1The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, Guangzhou, Guangdong, China; 2China Medical Team, Takeda Pharmaceutical Company, Shanghai, China, Shanghai, Shanghai, China; 3Takeda Pharmaceutical Company, Shanghai, China, Shanghai, Shanghai, China
Introduction: Vonoprazan, a potassium-competitive acid blocker, is approved in China as a first-line treatment for reflux esophagitis (RE). To better understand the profile of patients receiving vonoprazan in China, this post hoc analysis assessed the baseline characteristics of patients with RE treated with vonoprazan in the real-world VIEW study and the differences in characteristics based on age and disease severity.
Methods: VIEW (NCT04501627) was a multicenter, single-arm, prospective, observational, real-world study in China. Patients received 20 mg vonoprazan, orally, once daily for 4 weeks (8 weeks if insufficient healing), followed by a 2-week safety evaluation after treatment. In this post hoc analysis, differences in baseline characteristics and demographics were analyzed by age ( < 65 vs ≥65 years) and RE severity (mild vs severe).
Results: Of the 1877 patients included in the RE safety analysis population, mean (standard deviation [SD]) age was 49.7 (13.4) years; 64.4% were men, and mean (SD) body mass index was 24.06 (3.47) kg/m2. Most patients had never smoked (73.9%) and never consumed alcohol (73.4%). Los Angeles (LA) classification at baseline showed mild RE with 41.3% in grade A and 27.9% in grade B, and severe RE with 6.1% in grade C and 1.9% in grade D. Barrett’s mucosa was present in 2.5% of patients and esophageal ulceration in 12.8% of patients. Mean total gastroesophageal reflux disease questionnaire (GERDQ) score was 8.4 at baseline. The percentage of patients taking vonoprazan for 4 weeks and 8 weeks was 76.1% and 23.9%, respectively (Table 1). Patient characteristics that were significantly different by age ( < 65 vs ≥65 years) included gender (P=0.004), smoking status (P=0.003), alcohol consumption (P < 0.001), LA classification (P < 0.001), absence/presence of Barrett’s mucosa (P=0.005), and total GERDQ score (P=0.026) (Table 2). Patient characteristics that were significantly different by severity (mild vs severe) were age (P < 0.001); esophageal ulceration (P < 0.001); symptoms at enrollment, including all-day heartburn and regurgitation (P=0.014), nighttime heartburn (P < 0.001) and nighttime regurgitation (P=0.003); total GERDQ score (P=0.020); and vonoprazan treatment duration (P < 0.001) (Table 2).
Discussion: This post hoc analysis revealed significant variation in the key baseline characteristics of patients with RE treated with vonoprazan in China, based on age and disease severity.
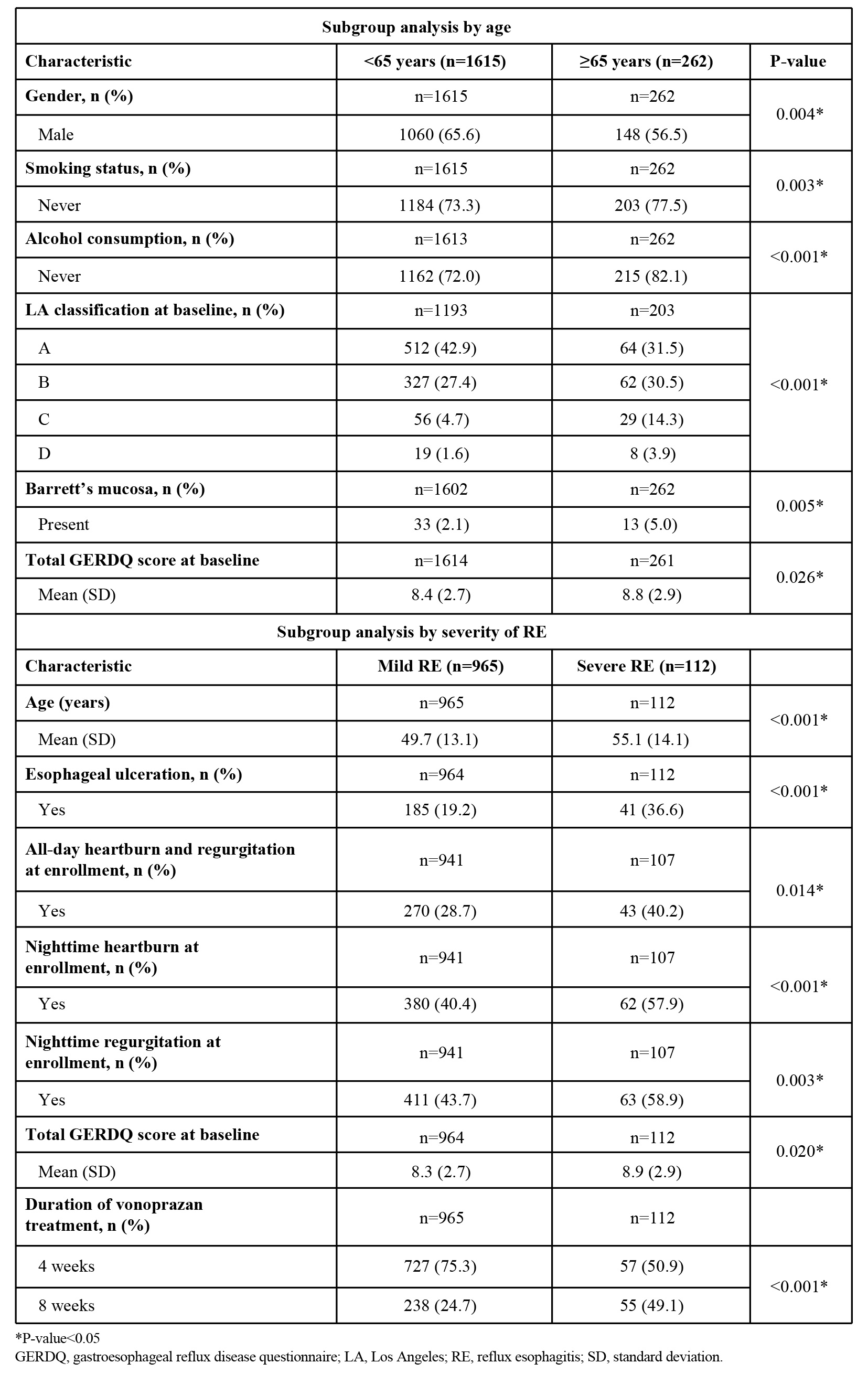
Figure: Table 1. Summary of demographic and baseline characteristics of patients with RE in the safety analysis population
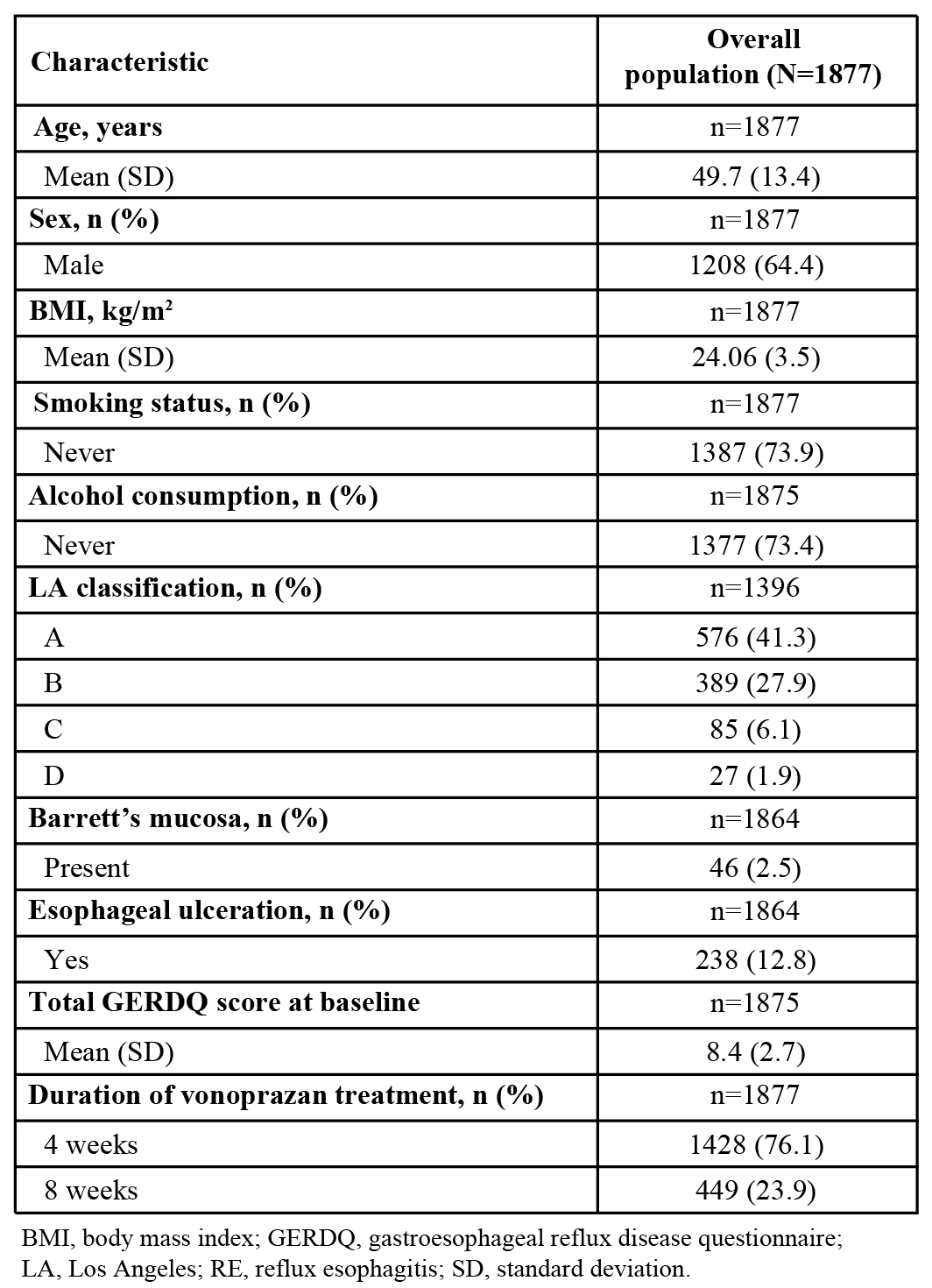
Figure: Table 2. Subgroup analysis: Significantly different demographics and baseline characteristics of patients with RE by age and severity of RE
Disclosures:
Yinglian Xiao: Takeda (China) International Trading Co. Ltd – Grant/Research Support.
Kailun Liang: Takeda (China) International Trading Co. Ltd – Employee, Stock Options.
Fang Zhou: Takeda (China) International Trading Co. Ltd – Employee, Stock Options.
Minhu Chen: AstraZeneca China – Speaker honoraria. Eisai China – Speaker honoraria. Takeda (China) International Trading Co. Ltd – Grant/Research Support, Royalties. Xian Janssen – Speaker honoraria.
Yinglian Xiao, MD, PhD1, Kailun Liang, MSc2, Fang Zhou, MSc3, Minhu Chen, MD, PhD1. P2775 - Baseline Characteristics of Patients With Reflux Esophagitis Receiving Vonoprazan Treatment in China: Post Hoc Analysis of the VIEW Real-World Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, Guangzhou, Guangdong, China; 2China Medical Team, Takeda Pharmaceutical Company, Shanghai, China, Shanghai, Shanghai, China; 3Takeda Pharmaceutical Company, Shanghai, China, Shanghai, Shanghai, China
Introduction: Vonoprazan, a potassium-competitive acid blocker, is approved in China as a first-line treatment for reflux esophagitis (RE). To better understand the profile of patients receiving vonoprazan in China, this post hoc analysis assessed the baseline characteristics of patients with RE treated with vonoprazan in the real-world VIEW study and the differences in characteristics based on age and disease severity.
Methods: VIEW (NCT04501627) was a multicenter, single-arm, prospective, observational, real-world study in China. Patients received 20 mg vonoprazan, orally, once daily for 4 weeks (8 weeks if insufficient healing), followed by a 2-week safety evaluation after treatment. In this post hoc analysis, differences in baseline characteristics and demographics were analyzed by age ( < 65 vs ≥65 years) and RE severity (mild vs severe).
Results: Of the 1877 patients included in the RE safety analysis population, mean (standard deviation [SD]) age was 49.7 (13.4) years; 64.4% were men, and mean (SD) body mass index was 24.06 (3.47) kg/m2. Most patients had never smoked (73.9%) and never consumed alcohol (73.4%). Los Angeles (LA) classification at baseline showed mild RE with 41.3% in grade A and 27.9% in grade B, and severe RE with 6.1% in grade C and 1.9% in grade D. Barrett’s mucosa was present in 2.5% of patients and esophageal ulceration in 12.8% of patients. Mean total gastroesophageal reflux disease questionnaire (GERDQ) score was 8.4 at baseline. The percentage of patients taking vonoprazan for 4 weeks and 8 weeks was 76.1% and 23.9%, respectively (Table 1). Patient characteristics that were significantly different by age ( < 65 vs ≥65 years) included gender (P=0.004), smoking status (P=0.003), alcohol consumption (P < 0.001), LA classification (P < 0.001), absence/presence of Barrett’s mucosa (P=0.005), and total GERDQ score (P=0.026) (Table 2). Patient characteristics that were significantly different by severity (mild vs severe) were age (P < 0.001); esophageal ulceration (P < 0.001); symptoms at enrollment, including all-day heartburn and regurgitation (P=0.014), nighttime heartburn (P < 0.001) and nighttime regurgitation (P=0.003); total GERDQ score (P=0.020); and vonoprazan treatment duration (P < 0.001) (Table 2).
Discussion: This post hoc analysis revealed significant variation in the key baseline characteristics of patients with RE treated with vonoprazan in China, based on age and disease severity.

Figure: Table 1. Summary of demographic and baseline characteristics of patients with RE in the safety analysis population

Figure: Table 2. Subgroup analysis: Significantly different demographics and baseline characteristics of patients with RE by age and severity of RE
Disclosures:
Yinglian Xiao: Takeda (China) International Trading Co. Ltd – Grant/Research Support.
Kailun Liang: Takeda (China) International Trading Co. Ltd – Employee, Stock Options.
Fang Zhou: Takeda (China) International Trading Co. Ltd – Employee, Stock Options.
Minhu Chen: AstraZeneca China – Speaker honoraria. Eisai China – Speaker honoraria. Takeda (China) International Trading Co. Ltd – Grant/Research Support, Royalties. Xian Janssen – Speaker honoraria.
Yinglian Xiao, MD, PhD1, Kailun Liang, MSc2, Fang Zhou, MSc3, Minhu Chen, MD, PhD1. P2775 - Baseline Characteristics of Patients With Reflux Esophagitis Receiving Vonoprazan Treatment in China: Post Hoc Analysis of the VIEW Real-World Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
