Sunday Poster Session
Category: Stomach and Spleen
P2053 - Effects of GLP-1RA Use on Upper GI Toxicity in Patients Receiving Platinum Analog-Based Chemotherapy
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Varun Vemulapalli, MD
McGovern Medical School at UTHealth
Houston, TX
Presenting Author(s)
Varun Vemulapalli, MD1, Carolina Cruz, MD2, Rachel Mortan, MD3, Nina Quirk, MD, MS4, Humberto R.. Nieves-Jiménez, MD5, Andrew T. Sullivan, MD6, Anirudha Chatterjee, MD7, Jacob Reitnauer, 3, Cristina Natha, MD1, Yinghong Wang, MD, PhD, MS2
1McGovern Medical School at UTHealth, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas at Houston, Houston, TX; 4University of Texas Health Sciences Center in Houston, Houston, TX; 5Baylor College of Medicine / MD Anderson Cancer Center, Houston, TX; 6McGovern Medical School at UTHealth Houston, Houston, TX; 7University of Texas Medical Branch, Galveston, TX
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RA), initially approved for type 2 diabetes mellitus (T2DM), gained popularity after their 2014 indication expansion to weight loss. With rising use, it is important to understand their impact in vulnerable populations, such as patients with cancer. GLP-1RA are known to increase the risk of upper gastrointestinal (GI) symptoms like nausea, vomiting, and gastroparesis, which are also common adverse effects of platinum-based chemotherapy. This study evaluates the GI side effects of GLP-1RA in those receiving platinum analog therapy and examines their impact on treatment.
Methods: This was a single center, retrospective study including patients who received platinum analog chemotherapy. Patients were divided into two groups based on concurrent GLP-1RA use. Chi-square and univariate analyses were used to compare groups and calculate odds ratios.
Results: A total of 326 patients were included with 61.3% patients receiving concurrent GLP-1RA. Most were white (69.3%) and female (52.1%) with a median age of 58.4 years. GI malignancies (24.6%) and stage III-IV cancers (67.5%) were most common. Overall incidence of upper GI toxicity was 77.3% (n=252). Of those with toxicity, 124 had GLP-1RA use and 126 did not. The GLP-1RA group was older (61 vs 55, p< 0.0001), had more stage III-IV disease (73.4% vs 61.6%, p=0.047), head and neck cancer (10.3% vs 4%, p=0.050), and higher rates of T2DM (98.4% vs 88.9%, p=0.002) and opioid use (38.1% vs 16.7%, p< 0.0001). This group also tolerated more platinum doses (2.5 vs 1, p=0.001). Early satiety was the only GI symptom significantly associated with GLP-1RA use. A trend toward increased hospitalization was observed (16.7% vs 8.7%, p=0.058). No significant endoscopic or histologic differences were found. Univariate analysis revealed hypothyroidism (p=0.010) and opioid use (p=0.017) were associated with the lower incidence of upper GI toxicity, and mortality was associated with genitourinary cancer (OR 2.7, p=0.005), stage III-IV disease (OR 17.6, p< 0.0001), opioid use (OR 4.2, p< 0.0001), and hospitalization (OR 3.0, p< 0.004) among patients with upper GI toxicity.
Discussion: This is the first study to evaluate GLP-1RA use in patients receiving platinum-based chemotherapy. Overall upper GI toxicity was not significantly worse with GLP-1RA use but early satiety and trend toward increased hospitalization were noted. Future studies should validate these findings and study interactions with other cancer therapies.
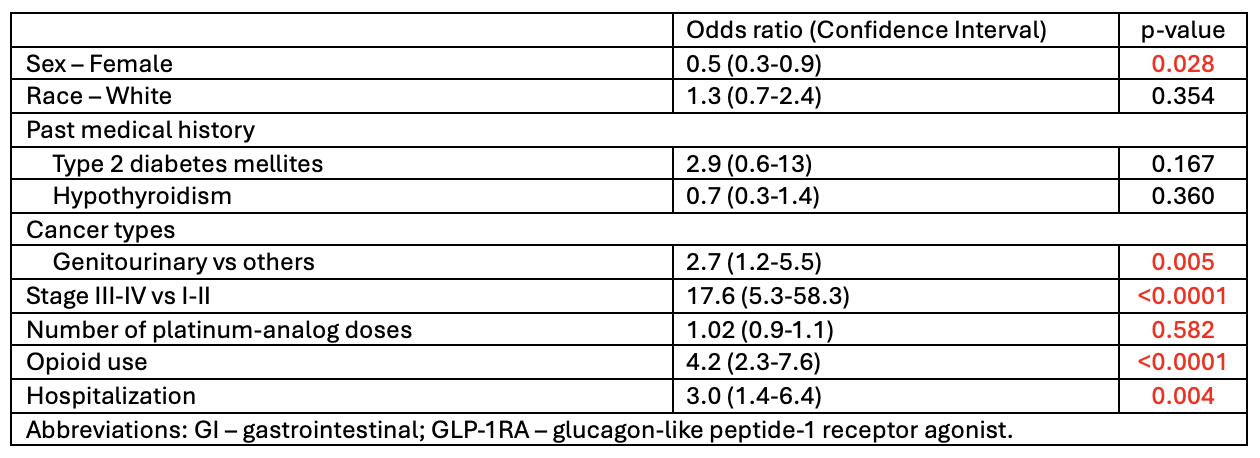
Figure: Univariate Analysis of Factors Associated with Mortality in Patients Who Developed Upper GI Toxicity While Receiving Platinum Analogs (n=252)
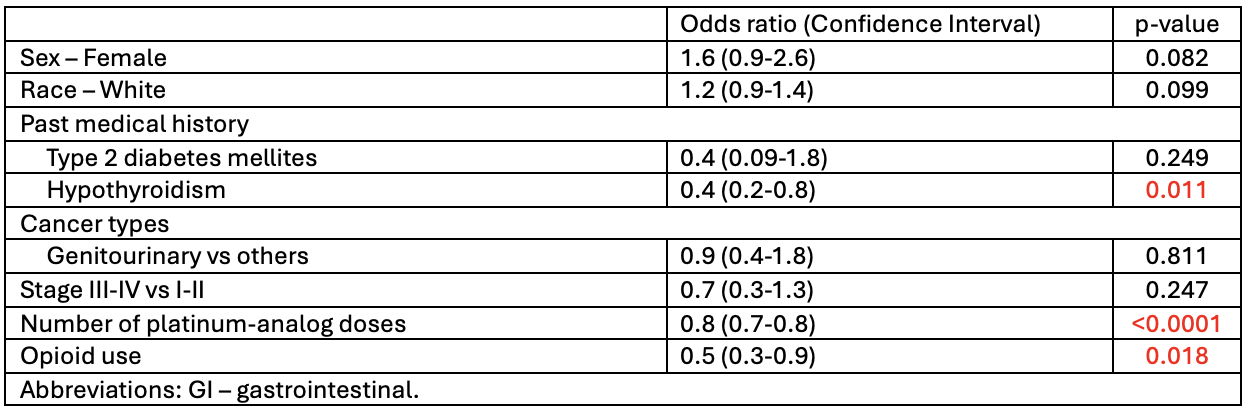
Figure: Univariate Analysis of Factors Associated with Upper GI Toxicity Among Patients Treated with Platinum Analogs (n=326)
Disclosures:
Varun Vemulapalli indicated no relevant financial relationships.
Carolina Cruz indicated no relevant financial relationships.
Rachel Mortan indicated no relevant financial relationships.
Nina Quirk indicated no relevant financial relationships.
Humberto Nieves-Jiménez indicated no relevant financial relationships.
Andrew Sullivan indicated no relevant financial relationships.
Anirudha Chatterjee indicated no relevant financial relationships.
Jacob Reitnauer indicated no relevant financial relationships.
Cristina Natha indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Varun Vemulapalli, MD1, Carolina Cruz, MD2, Rachel Mortan, MD3, Nina Quirk, MD, MS4, Humberto R.. Nieves-Jiménez, MD5, Andrew T. Sullivan, MD6, Anirudha Chatterjee, MD7, Jacob Reitnauer, 3, Cristina Natha, MD1, Yinghong Wang, MD, PhD, MS2. P2053 - Effects of GLP-1RA Use on Upper GI Toxicity in Patients Receiving Platinum Analog-Based Chemotherapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1McGovern Medical School at UTHealth, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas at Houston, Houston, TX; 4University of Texas Health Sciences Center in Houston, Houston, TX; 5Baylor College of Medicine / MD Anderson Cancer Center, Houston, TX; 6McGovern Medical School at UTHealth Houston, Houston, TX; 7University of Texas Medical Branch, Galveston, TX
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RA), initially approved for type 2 diabetes mellitus (T2DM), gained popularity after their 2014 indication expansion to weight loss. With rising use, it is important to understand their impact in vulnerable populations, such as patients with cancer. GLP-1RA are known to increase the risk of upper gastrointestinal (GI) symptoms like nausea, vomiting, and gastroparesis, which are also common adverse effects of platinum-based chemotherapy. This study evaluates the GI side effects of GLP-1RA in those receiving platinum analog therapy and examines their impact on treatment.
Methods: This was a single center, retrospective study including patients who received platinum analog chemotherapy. Patients were divided into two groups based on concurrent GLP-1RA use. Chi-square and univariate analyses were used to compare groups and calculate odds ratios.
Results: A total of 326 patients were included with 61.3% patients receiving concurrent GLP-1RA. Most were white (69.3%) and female (52.1%) with a median age of 58.4 years. GI malignancies (24.6%) and stage III-IV cancers (67.5%) were most common. Overall incidence of upper GI toxicity was 77.3% (n=252). Of those with toxicity, 124 had GLP-1RA use and 126 did not. The GLP-1RA group was older (61 vs 55, p< 0.0001), had more stage III-IV disease (73.4% vs 61.6%, p=0.047), head and neck cancer (10.3% vs 4%, p=0.050), and higher rates of T2DM (98.4% vs 88.9%, p=0.002) and opioid use (38.1% vs 16.7%, p< 0.0001). This group also tolerated more platinum doses (2.5 vs 1, p=0.001). Early satiety was the only GI symptom significantly associated with GLP-1RA use. A trend toward increased hospitalization was observed (16.7% vs 8.7%, p=0.058). No significant endoscopic or histologic differences were found. Univariate analysis revealed hypothyroidism (p=0.010) and opioid use (p=0.017) were associated with the lower incidence of upper GI toxicity, and mortality was associated with genitourinary cancer (OR 2.7, p=0.005), stage III-IV disease (OR 17.6, p< 0.0001), opioid use (OR 4.2, p< 0.0001), and hospitalization (OR 3.0, p< 0.004) among patients with upper GI toxicity.
Discussion: This is the first study to evaluate GLP-1RA use in patients receiving platinum-based chemotherapy. Overall upper GI toxicity was not significantly worse with GLP-1RA use but early satiety and trend toward increased hospitalization were noted. Future studies should validate these findings and study interactions with other cancer therapies.

Figure: Univariate Analysis of Factors Associated with Mortality in Patients Who Developed Upper GI Toxicity While Receiving Platinum Analogs (n=252)

Figure: Univariate Analysis of Factors Associated with Upper GI Toxicity Among Patients Treated with Platinum Analogs (n=326)
Disclosures:
Varun Vemulapalli indicated no relevant financial relationships.
Carolina Cruz indicated no relevant financial relationships.
Rachel Mortan indicated no relevant financial relationships.
Nina Quirk indicated no relevant financial relationships.
Humberto Nieves-Jiménez indicated no relevant financial relationships.
Andrew Sullivan indicated no relevant financial relationships.
Anirudha Chatterjee indicated no relevant financial relationships.
Jacob Reitnauer indicated no relevant financial relationships.
Cristina Natha indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Varun Vemulapalli, MD1, Carolina Cruz, MD2, Rachel Mortan, MD3, Nina Quirk, MD, MS4, Humberto R.. Nieves-Jiménez, MD5, Andrew T. Sullivan, MD6, Anirudha Chatterjee, MD7, Jacob Reitnauer, 3, Cristina Natha, MD1, Yinghong Wang, MD, PhD, MS2. P2053 - Effects of GLP-1RA Use on Upper GI Toxicity in Patients Receiving Platinum Analog-Based Chemotherapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
