Sunday Poster Session
Category: Stomach and Spleen
P2049 - Recurrent Upper Gastrointestinal Immune-Related Adverse Events: Clinical Features and Outcomes
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Maria Julia M. N Santos, MD (she/her/hers)
MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Maria Julia M. N.. Santos, MD1, Carolina Cruz, MD2, Reema Patel, MD3, Anirudha Chatterjee, MD3, Sharada Wali, MBBS, MPH2, Andrew Lee, BS2, Hari Movva, MD3, Mehnaz Shafi, MD1, Anusha Shirwaikar Thomas, MD2, Krishnavathana Varatharajalu, MD1, Yinghong Wang, MD, PhD, MS2
1MD Anderson Cancer Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas Medical Branch, Galveston, TX
Introduction: Upper gastrointestinal (GI) immune-related adverse events have been reported more frequently in recent years, due to the broader use of immune checkpoint inhibitors (ICIs) as an anti-cancer therapy. While most irAEs resolve with conservative management, there are increasing instances of recurrences following clinical or endoscopic remission, which can significantly affect the quality of life for those affected. As this is a relatively new entity, its characteristics, clinical course, and outcomes are still not well-defined. It is crucial to better understand this presentation to improve surveillance and inform therapeutic decision-making for patients undergoing ICI therapy.
Methods: We retrospectively identified 41 patients who developed recurrent upper GI irAEs ≥ 2 months up to 40 months after initial toxicity resolution (clinical and/or endoscopic remission). Data on presentation, management, and outcomes were summarized descriptively. Additionally, we performed univariate analyses in a broader cohort of 529 patients to identify predictors of recurrence.
Results: Among the 41 patients with recurrence, most were initially treated with PD-1/L1 inhibitors (80.5%), while 19.5% received combination ICI. The median time from first event to recurrence was 9.4 months (IQR 4.2–19.2), and CTCAE grade 3 toxicity was reported in 36.6% of patients. Among the recurrence findings, gastritis (48.8%) and esophagitis with gastroenteritis (19.5%) were the most frequent. Management was primarily supportive (73.2%), with 17.1% receiving vedolizumab and 4.8% receiving steroids. Resolution of symptoms after recurrence was achieved in 73.2%, while 26.8% had persistent symptoms at last follow-up. In univariate analysis, predictors of recurrence included overlap of GI segments at initial toxicity (OR 3.86, p=0.001), steroid use (OR 2.44, p=0.007), selective immunosuppressive therapy (SIT) use (OR 2.73, p=0.004), and continuation of ICI therapy (OR 2.18, p=0.019).
Discussion: Recurrent upper GI irAEs frequently mirror the clinical presentation and severity of the initial episode but are often managed conservatively, without escalation to systemic immunosuppression. Despite this, over one-quarter of patients experience persistent symptoms, particularly those with overlapping GI involvement, prior steroid or SIT use, or continued ICI therapy. Incorporating clinical risk factors into follow-up strategies may improve outcomes, and these results underscore the need for close follow-up after resolution of first event.
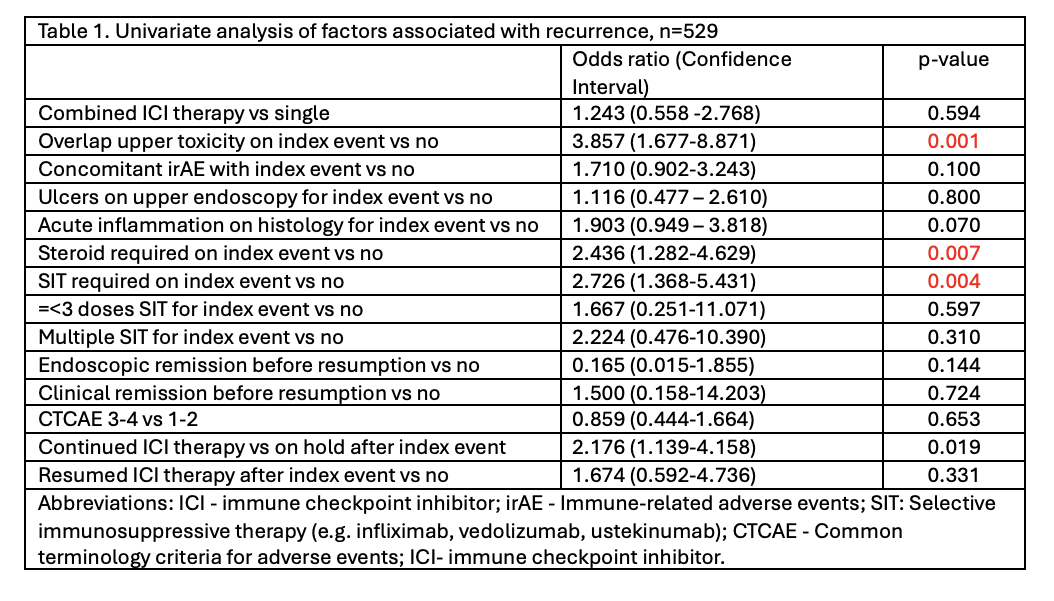
Figure: Table 1. Univariate analysis of factors associated with recurrence
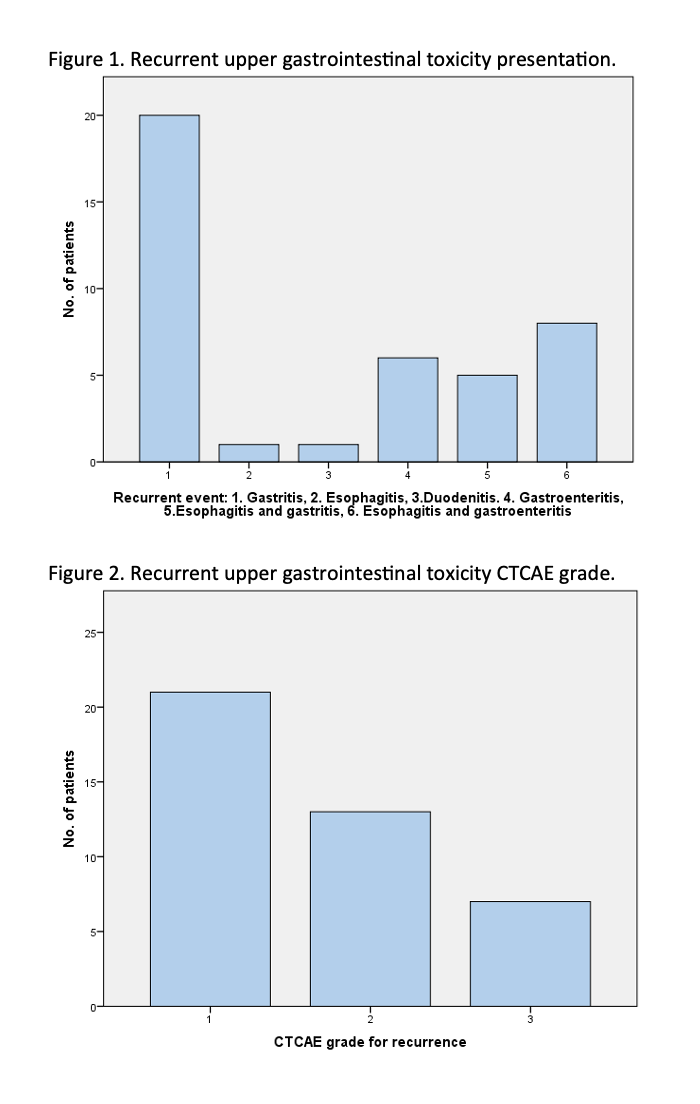
Figure: Recurrent upper gastrointestinal toxicity presentation and CTCAE grade.
Disclosures:
Maria Julia Santos indicated no relevant financial relationships.
Carolina Cruz indicated no relevant financial relationships.
Reema Patel indicated no relevant financial relationships.
Anirudha Chatterjee indicated no relevant financial relationships.
Sharada Wali indicated no relevant financial relationships.
Andrew Lee indicated no relevant financial relationships.
Hari Movva indicated no relevant financial relationships.
Mehnaz Shafi indicated no relevant financial relationships.
Anusha Shirwaikar Thomas indicated no relevant financial relationships.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Maria Julia M. N.. Santos, MD1, Carolina Cruz, MD2, Reema Patel, MD3, Anirudha Chatterjee, MD3, Sharada Wali, MBBS, MPH2, Andrew Lee, BS2, Hari Movva, MD3, Mehnaz Shafi, MD1, Anusha Shirwaikar Thomas, MD2, Krishnavathana Varatharajalu, MD1, Yinghong Wang, MD, PhD, MS2. P2049 - Recurrent Upper Gastrointestinal Immune-Related Adverse Events: Clinical Features and Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1MD Anderson Cancer Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas Medical Branch, Galveston, TX
Introduction: Upper gastrointestinal (GI) immune-related adverse events have been reported more frequently in recent years, due to the broader use of immune checkpoint inhibitors (ICIs) as an anti-cancer therapy. While most irAEs resolve with conservative management, there are increasing instances of recurrences following clinical or endoscopic remission, which can significantly affect the quality of life for those affected. As this is a relatively new entity, its characteristics, clinical course, and outcomes are still not well-defined. It is crucial to better understand this presentation to improve surveillance and inform therapeutic decision-making for patients undergoing ICI therapy.
Methods: We retrospectively identified 41 patients who developed recurrent upper GI irAEs ≥ 2 months up to 40 months after initial toxicity resolution (clinical and/or endoscopic remission). Data on presentation, management, and outcomes were summarized descriptively. Additionally, we performed univariate analyses in a broader cohort of 529 patients to identify predictors of recurrence.
Results: Among the 41 patients with recurrence, most were initially treated with PD-1/L1 inhibitors (80.5%), while 19.5% received combination ICI. The median time from first event to recurrence was 9.4 months (IQR 4.2–19.2), and CTCAE grade 3 toxicity was reported in 36.6% of patients. Among the recurrence findings, gastritis (48.8%) and esophagitis with gastroenteritis (19.5%) were the most frequent. Management was primarily supportive (73.2%), with 17.1% receiving vedolizumab and 4.8% receiving steroids. Resolution of symptoms after recurrence was achieved in 73.2%, while 26.8% had persistent symptoms at last follow-up. In univariate analysis, predictors of recurrence included overlap of GI segments at initial toxicity (OR 3.86, p=0.001), steroid use (OR 2.44, p=0.007), selective immunosuppressive therapy (SIT) use (OR 2.73, p=0.004), and continuation of ICI therapy (OR 2.18, p=0.019).
Discussion: Recurrent upper GI irAEs frequently mirror the clinical presentation and severity of the initial episode but are often managed conservatively, without escalation to systemic immunosuppression. Despite this, over one-quarter of patients experience persistent symptoms, particularly those with overlapping GI involvement, prior steroid or SIT use, or continued ICI therapy. Incorporating clinical risk factors into follow-up strategies may improve outcomes, and these results underscore the need for close follow-up after resolution of first event.

Figure: Table 1. Univariate analysis of factors associated with recurrence

Figure: Recurrent upper gastrointestinal toxicity presentation and CTCAE grade.
Disclosures:
Maria Julia Santos indicated no relevant financial relationships.
Carolina Cruz indicated no relevant financial relationships.
Reema Patel indicated no relevant financial relationships.
Anirudha Chatterjee indicated no relevant financial relationships.
Sharada Wali indicated no relevant financial relationships.
Andrew Lee indicated no relevant financial relationships.
Hari Movva indicated no relevant financial relationships.
Mehnaz Shafi indicated no relevant financial relationships.
Anusha Shirwaikar Thomas indicated no relevant financial relationships.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Maria Julia M. N.. Santos, MD1, Carolina Cruz, MD2, Reema Patel, MD3, Anirudha Chatterjee, MD3, Sharada Wali, MBBS, MPH2, Andrew Lee, BS2, Hari Movva, MD3, Mehnaz Shafi, MD1, Anusha Shirwaikar Thomas, MD2, Krishnavathana Varatharajalu, MD1, Yinghong Wang, MD, PhD, MS2. P2049 - Recurrent Upper Gastrointestinal Immune-Related Adverse Events: Clinical Features and Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
