Sunday Poster Session
Category: Stomach and Spleen
P2035 - Comparative Safety of the Novel Potassium-Competitive Acid Blocker Vonoprazan vs Proton Pump Inhibitors: A Pharmacovigilance Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Ratnadeep Biswas, MBBS (he/him/his)
ESIC Medical College & Hospital, Bihta, Patna
Patna, Bihar, India
Presenting Author(s)
Award: ACG Presidential Poster Award
Ratnadeep Biswas, MBBS1, Pratima Jasti, MBBS2, Farheen F.. Vhora, MBBS3, Abhay A.. Kapoor, MBBS4, Jaahnavee Trivedi, MBBS5
1ESIC Medical College & Hospital, Bihta, Patna, Patna, Bihar, India; 2All India Institute of Medical Sciences, Bhubaneswar, Bhubaneswar, Orissa, India; 3B.J. Medical College, Ahmedabad, Ahmedabad, Gujarat, India; 4St. Mary Medical Center, Langhorne, Langhorne, PA; 5SUNY Downstate Health Sciences University, Brooklyn, NY
Introduction: Vonoprazan, a novel potassium-competitive acid blocker (P-CAB), has emerged as an alternative to proton pump inhibitors (PPIs) for acid-related conditions. Despite its increasing use, real-world safety data comparing vonoprazan to PPIs remain limited. This study aimed to evaluate and compare adverse event (AE) profiles of vonoprazan vs. PPIs using data from a large post-marketing safety database.
Methods: We queried the FDA Adverse Event Reporting System (FAERS) database for AEs reported for vonoprazan and the three most commonly used PPIs (pantoprazole, omeprazole and esomeprazole) between 2023-25. AEs were categorized according to seriousness and system organ class (SOC). Univariate logistic regression analyses were used to compare event rates between vonoprazan and PPIs.
Results: Out of 2581 reports, 524 AEs were reported for vonoprazan while 2057 for PPIs. Vonoprazan was associated with a significantly lower proportion of serious AEs compared to PPIs (38.9% vs. 89.3%, p< 0.001), with a markedly lower odds of serious events (OR 0.077, 95% CI: 0.061–0.096). Death (2.9% vs. 14.4%, OR 0.18), life-threatening events (3.4% vs. 10.0%, OR 0.32), and disability (2.9% vs. 15.8%, OR 0.16) were significantly less frequent in vonoprazan reports (all p< 0.001).
Additionally, common gastrointestinal and neurological symptoms were less common with vonoprazan: diarrhea (5.9% vs. 30.4%, OR 0.14), nausea/vomiting (6.7% vs. 30.1%, OR 0.17), headache (5.5% vs. 26.3%, OR 0.16), and fatigue (6.5% vs. 28.2%, OR 0.18) (all p< 0.001). SOC-based analysis revealed significantly lower odds of AEs involving the gastrointestinal (OR 0.15), cardiovascular (OR 0.20), central nervous (OR 0.30), respiratory (OR 0.20), renal/urinary (OR 0.11), endocrine (OR 0.15), and other systems among vonoprazan users (all p< 0.001). Notably, however, urinary tract infections were more frequently reported with vonoprazan (OR 3.06, 95% CI: 1.33–7.02, p = 0.008).
Discussion: In this large pharmacovigilance-based comparison, vonoprazan demonstrated a substantially safer AE profile than PPIs across multiple domains, including serious outcomes and system-specific reactions. These findings suggest vonoprazan may offer a favorable safety alternative to PPIs for acid suppression therapy, warranting further prospective evaluation and paving the way for their more widespread use.
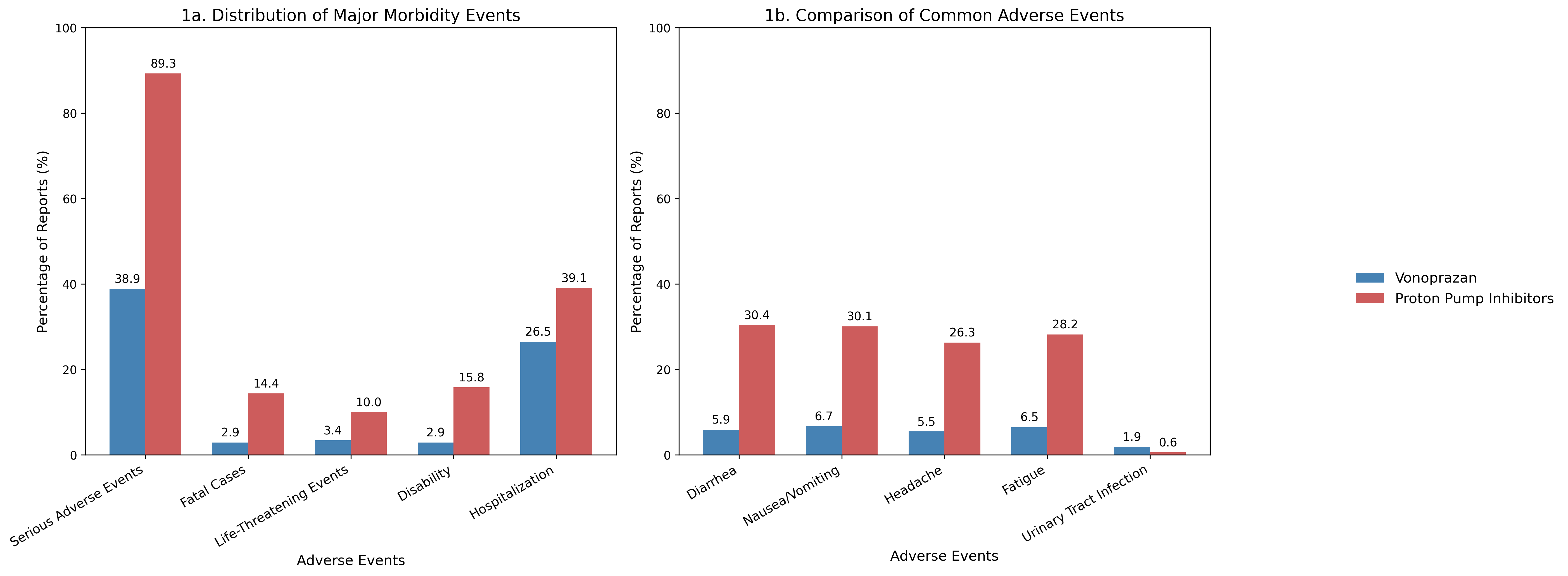
Figure: Graphs in Panels A and B compare adverse events of vonoprazan versus proton Pump Inhibitors; Panel A shows the distribution of major morbidity events, while Panel B presents the frequency of common adverse events
Disclosures:
Ratnadeep Biswas indicated no relevant financial relationships.
Pratima Jasti indicated no relevant financial relationships.
Farheen Vhora indicated no relevant financial relationships.
Abhay Kapoor indicated no relevant financial relationships.
Jaahnavee Trivedi indicated no relevant financial relationships.
Ratnadeep Biswas, MBBS1, Pratima Jasti, MBBS2, Farheen F.. Vhora, MBBS3, Abhay A.. Kapoor, MBBS4, Jaahnavee Trivedi, MBBS5. P2035 - Comparative Safety of the Novel Potassium-Competitive Acid Blocker Vonoprazan vs Proton Pump Inhibitors: A Pharmacovigilance Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Ratnadeep Biswas, MBBS1, Pratima Jasti, MBBS2, Farheen F.. Vhora, MBBS3, Abhay A.. Kapoor, MBBS4, Jaahnavee Trivedi, MBBS5
1ESIC Medical College & Hospital, Bihta, Patna, Patna, Bihar, India; 2All India Institute of Medical Sciences, Bhubaneswar, Bhubaneswar, Orissa, India; 3B.J. Medical College, Ahmedabad, Ahmedabad, Gujarat, India; 4St. Mary Medical Center, Langhorne, Langhorne, PA; 5SUNY Downstate Health Sciences University, Brooklyn, NY
Introduction: Vonoprazan, a novel potassium-competitive acid blocker (P-CAB), has emerged as an alternative to proton pump inhibitors (PPIs) for acid-related conditions. Despite its increasing use, real-world safety data comparing vonoprazan to PPIs remain limited. This study aimed to evaluate and compare adverse event (AE) profiles of vonoprazan vs. PPIs using data from a large post-marketing safety database.
Methods: We queried the FDA Adverse Event Reporting System (FAERS) database for AEs reported for vonoprazan and the three most commonly used PPIs (pantoprazole, omeprazole and esomeprazole) between 2023-25. AEs were categorized according to seriousness and system organ class (SOC). Univariate logistic regression analyses were used to compare event rates between vonoprazan and PPIs.
Results: Out of 2581 reports, 524 AEs were reported for vonoprazan while 2057 for PPIs. Vonoprazan was associated with a significantly lower proportion of serious AEs compared to PPIs (38.9% vs. 89.3%, p< 0.001), with a markedly lower odds of serious events (OR 0.077, 95% CI: 0.061–0.096). Death (2.9% vs. 14.4%, OR 0.18), life-threatening events (3.4% vs. 10.0%, OR 0.32), and disability (2.9% vs. 15.8%, OR 0.16) were significantly less frequent in vonoprazan reports (all p< 0.001).
Additionally, common gastrointestinal and neurological symptoms were less common with vonoprazan: diarrhea (5.9% vs. 30.4%, OR 0.14), nausea/vomiting (6.7% vs. 30.1%, OR 0.17), headache (5.5% vs. 26.3%, OR 0.16), and fatigue (6.5% vs. 28.2%, OR 0.18) (all p< 0.001). SOC-based analysis revealed significantly lower odds of AEs involving the gastrointestinal (OR 0.15), cardiovascular (OR 0.20), central nervous (OR 0.30), respiratory (OR 0.20), renal/urinary (OR 0.11), endocrine (OR 0.15), and other systems among vonoprazan users (all p< 0.001). Notably, however, urinary tract infections were more frequently reported with vonoprazan (OR 3.06, 95% CI: 1.33–7.02, p = 0.008).
Discussion: In this large pharmacovigilance-based comparison, vonoprazan demonstrated a substantially safer AE profile than PPIs across multiple domains, including serious outcomes and system-specific reactions. These findings suggest vonoprazan may offer a favorable safety alternative to PPIs for acid suppression therapy, warranting further prospective evaluation and paving the way for their more widespread use.

Figure: Graphs in Panels A and B compare adverse events of vonoprazan versus proton Pump Inhibitors; Panel A shows the distribution of major morbidity events, while Panel B presents the frequency of common adverse events
Disclosures:
Ratnadeep Biswas indicated no relevant financial relationships.
Pratima Jasti indicated no relevant financial relationships.
Farheen Vhora indicated no relevant financial relationships.
Abhay Kapoor indicated no relevant financial relationships.
Jaahnavee Trivedi indicated no relevant financial relationships.
Ratnadeep Biswas, MBBS1, Pratima Jasti, MBBS2, Farheen F.. Vhora, MBBS3, Abhay A.. Kapoor, MBBS4, Jaahnavee Trivedi, MBBS5. P2035 - Comparative Safety of the Novel Potassium-Competitive Acid Blocker Vonoprazan vs Proton Pump Inhibitors: A Pharmacovigilance Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

