Monday Poster Session
Category: Biliary/Pancreas
P2166 - GLP-1 Receptor Agonists Are Associated With Significant Protection From Acute Pancreatitis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Inuk Zandvakili, MD, PhD
University of Cincinnati
Cincinnati, OH
Presenting Author(s)
Inuk Zandvakili, MD, PhD, Wei-Wen Hsu, PhD, Luis Lara, MD
University of Cincinnati, Cincinnati, OH
Introduction: Acute pancreatitis (AP) is a common cause of hospital admission resulting in significant morbidity and mortality. Pancreatic islet β-cells have receptors for GLP-1 and initial clinical trials of GLP-1 receptor agonists (GLP1RA) for the treatment of diabetes mellitus found increased rates of AP resulting in FDA warnings in package inserts.
Methods: Here, we utilize the ultra-large clinical database TriNetX and find that GLP1RA is associated with lower rates of AP. Specifically, we searched for adults age ≥18 years who had ICD codes for either diabetes mellitus and were overweight or obese, and did not have prior pancreatitis, cystic fibrosis, pancreatic neoplasms, cysts or surgeries, among other exclusion codes. We assessed the outcome of AP over a 10-year period utilizing propensity score matching (PSM) to compare those taking a GLP1RA for ≥1 year (n = 82,902) versus those not taking GLP1RA (n = ~7.4 million), using ATC codes. After PSM, all 82,902 individuals in the GLP1RA group were matched to those not exposed to GLP1RA.
Results: For the outcome of AP, approximately 5,000 individuals were excluded due to AP prior to the 10-year time window, and we found ~80% reduction in incidence of AP associated with GLP1RA exposure. We also assessed for severe AP, defined as AP plus acute kidney injury, respiratory failure, hypovolemic shock or endotracheal intubation and found an ~80% reduction in severe AP associated with GLP1RA exposure. We also assessed for ICD codes for chronic pancreatitis (CP) and found a ~78% reduction associated with GLP1RA exposure. Finally, we assessed outcomes of CPT codes for either ERCP and EUS and found a ~76% and 61% risk reduction associated with GLP1RA exposure, respectively.
Discussion: In summary, using real world data utilizing diagnosis, medication and procedure codes from TriNetX, we found GLP1RA exposure associated with decreases in acute and chronic pancreatitis, ERCP and EUS. Strengths of this study include use of real-world data, large study cohort, stringent exclusion criteria and no dropouts with PSM. Limitations of this study include the use of diagnosis codes and inability to verify drug exposure. Long term clinical trials of GLP1RA use for cardiovascular outcomes and safety are ongoing and will confirm the validity of these findings.
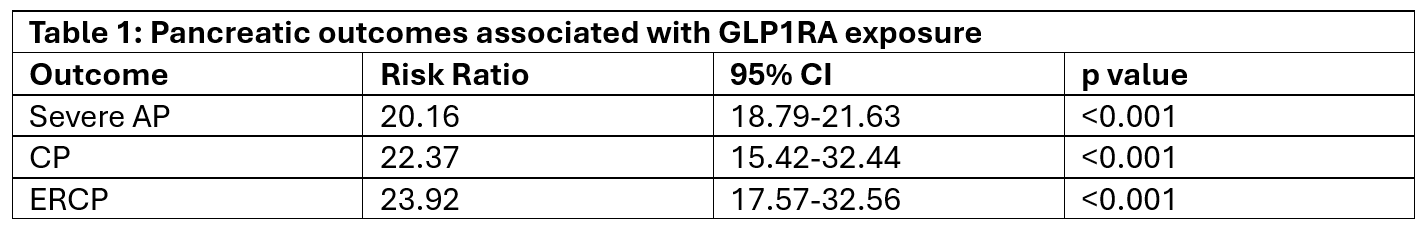
Figure: Table 1: Pancreatic outcomes associated with GLP1RA exposure
Disclosures:
Inuk Zandvakili: Eli Lilly – Consultant.
Wei-Wen Hsu indicated no relevant financial relationships.
Luis Lara: AbbVie – Consultant, Grant/Research Support, Speakers Bureau.
Inuk Zandvakili, MD, PhD, Wei-Wen Hsu, PhD, Luis Lara, MD. P2166 - GLP-1 Receptor Agonists Are Associated With Significant Protection From Acute Pancreatitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Cincinnati, Cincinnati, OH
Introduction: Acute pancreatitis (AP) is a common cause of hospital admission resulting in significant morbidity and mortality. Pancreatic islet β-cells have receptors for GLP-1 and initial clinical trials of GLP-1 receptor agonists (GLP1RA) for the treatment of diabetes mellitus found increased rates of AP resulting in FDA warnings in package inserts.
Methods: Here, we utilize the ultra-large clinical database TriNetX and find that GLP1RA is associated with lower rates of AP. Specifically, we searched for adults age ≥18 years who had ICD codes for either diabetes mellitus and were overweight or obese, and did not have prior pancreatitis, cystic fibrosis, pancreatic neoplasms, cysts or surgeries, among other exclusion codes. We assessed the outcome of AP over a 10-year period utilizing propensity score matching (PSM) to compare those taking a GLP1RA for ≥1 year (n = 82,902) versus those not taking GLP1RA (n = ~7.4 million), using ATC codes. After PSM, all 82,902 individuals in the GLP1RA group were matched to those not exposed to GLP1RA.
Results: For the outcome of AP, approximately 5,000 individuals were excluded due to AP prior to the 10-year time window, and we found ~80% reduction in incidence of AP associated with GLP1RA exposure. We also assessed for severe AP, defined as AP plus acute kidney injury, respiratory failure, hypovolemic shock or endotracheal intubation and found an ~80% reduction in severe AP associated with GLP1RA exposure. We also assessed for ICD codes for chronic pancreatitis (CP) and found a ~78% reduction associated with GLP1RA exposure. Finally, we assessed outcomes of CPT codes for either ERCP and EUS and found a ~76% and 61% risk reduction associated with GLP1RA exposure, respectively.
Discussion: In summary, using real world data utilizing diagnosis, medication and procedure codes from TriNetX, we found GLP1RA exposure associated with decreases in acute and chronic pancreatitis, ERCP and EUS. Strengths of this study include use of real-world data, large study cohort, stringent exclusion criteria and no dropouts with PSM. Limitations of this study include the use of diagnosis codes and inability to verify drug exposure. Long term clinical trials of GLP1RA use for cardiovascular outcomes and safety are ongoing and will confirm the validity of these findings.

Figure: Table 1: Pancreatic outcomes associated with GLP1RA exposure
Disclosures:
Inuk Zandvakili: Eli Lilly – Consultant.
Wei-Wen Hsu indicated no relevant financial relationships.
Luis Lara: AbbVie – Consultant, Grant/Research Support, Speakers Bureau.
Inuk Zandvakili, MD, PhD, Wei-Wen Hsu, PhD, Luis Lara, MD. P2166 - GLP-1 Receptor Agonists Are Associated With Significant Protection From Acute Pancreatitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
