Monday Poster Session
Category: Biliary/Pancreas
P2234 - Indomethacin Does Not Reduce Risk of Post-ERCP Acute Pancreatitis Compared to Other NSAIDs: A Propensity Score-Matched Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- PY
Pradeep Yarra, MD (he/him/his)
Saint Louis University School of Medicine
Saint Louis, MO
Presenting Author(s)
Pradeep Yarra, MD1, Yassine Kilani, MD1, Shravya R. Ginnaram, MD2, Ahmad Basil Nasir, MD1, Daniel Alejandro. Gonzalez Mosquera, MD3, Alfred Nelson, MD1, Wissam Kiwan, MD1
1Saint Louis University School of Medicine, Saint Louis, MO; 2University of Nebraska Medical Center, Omaha, NE; 3NYC Health + Hospitals/Lincoln, Bronx, NY
Introduction: Rectal indomethacin in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) has been shown to significantly reduce the incidence of post-ERCP pancreatitis. However, there is a lack of clinical studies comparing indomethacin to other oral non-steroid anti-inflammatory drugs (NSAIDs). This study aims to address this knowledge gap by evaluating the incidence of post-ERCP pancreatitis in patients who received indomethacin compared to those who received alternative NSAIDs (acetic, phenyl acetic and propionic acid derivatives, coxibs, oxicams) within three days before the procedure.
Methods: We conducted a retrospective cohort study in the United States using the TriNetX database to identify adults (≥18 years) undergoing ERCP and treated with indomethacin, excluding those with gallstone pancreatitis. This cohort was matched with patients undergoing ERCP and treated with other NSAIDs intake using 1:1 propensity score matching. The primary outcome was the incidence of post-ERCP pancreatitis within 7 days after ERCP, while the secondary outcome was the 7-day mortality. With censoring applied, Kaplan-Meier analysis with hazard ratios (HRs) and 95% CIs were used to compare time-to-event rates at daily time intervals.
Results: A total of 49,375 patients undergoing ERCP and treated with indomethacin were identified and propensity matched with 15,310 patients with AUD treated with other NSAIDs, resulting in 14,716 matched pairs. Patients treated with indomethacin were older, and had lower rates of pancreatitis risk factors (p < 0.001) as compared to controls (Table 1). Patients undergoing ERCP who received indomethacin had no significant differences in the risk of post-ERCP pancreatitis (1.6% vs. 1.3%; adjusted hazard ratio (aHR) = 1.21, 95% CI: 0.98–1.41), and mortality (0.4% vs. 0.5%, aHR = 0.87, 95%CI: 0.62 – 1.21) at 1 week follow-up (Table 2).
Discussion: Our findings provide real-world evidence that rectal indomethacin use in patients undergoing ERCP does not significantly reduce the risk of post-ERCP pancreatitis or mortality compared to other NSAIDs. Further prospective studies are needed to evaluate the benefits of other NSAIDs in prevention on post-ERCP pancreatitis, specifically in high risk patients and cost effectiveness.
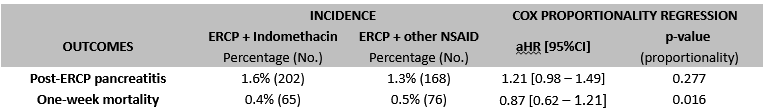
Figure: Baseline characteristics, data will be provided on sex/race/ethnicity/alcohol use, BMI and drugs: Non significant after matching
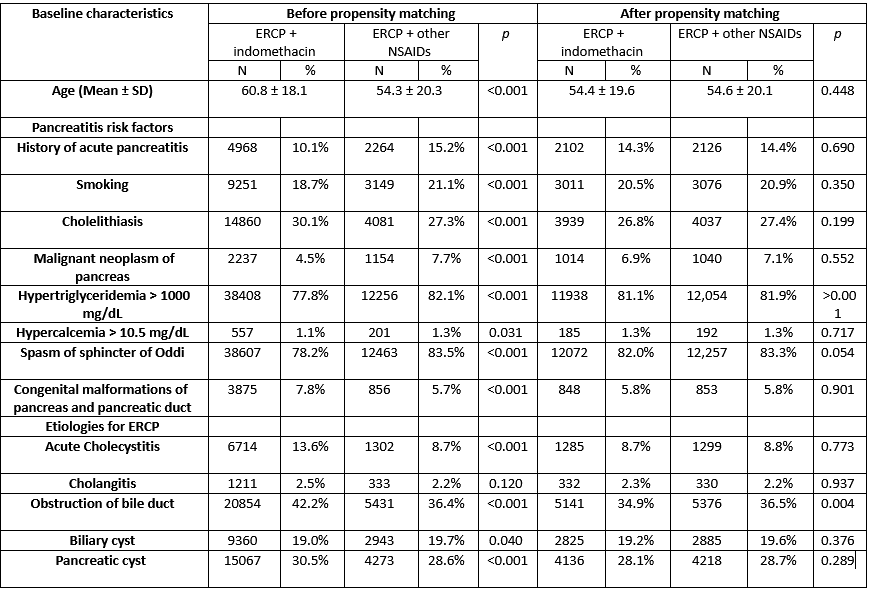
Figure: Incidence of post ERCP pancreatitis
Disclosures:
Pradeep Yarra indicated no relevant financial relationships.
Yassine Kilani indicated no relevant financial relationships.
Shravya Ginnaram indicated no relevant financial relationships.
Ahmad Basil Nasir indicated no relevant financial relationships.
Daniel Gonzalez Mosquera indicated no relevant financial relationships.
Alfred Nelson indicated no relevant financial relationships.
Wissam Kiwan indicated no relevant financial relationships.
Pradeep Yarra, MD1, Yassine Kilani, MD1, Shravya R. Ginnaram, MD2, Ahmad Basil Nasir, MD1, Daniel Alejandro. Gonzalez Mosquera, MD3, Alfred Nelson, MD1, Wissam Kiwan, MD1. P2234 - Indomethacin Does Not Reduce Risk of Post-ERCP Acute Pancreatitis Compared to Other NSAIDs: A Propensity Score-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Saint Louis University School of Medicine, Saint Louis, MO; 2University of Nebraska Medical Center, Omaha, NE; 3NYC Health + Hospitals/Lincoln, Bronx, NY
Introduction: Rectal indomethacin in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) has been shown to significantly reduce the incidence of post-ERCP pancreatitis. However, there is a lack of clinical studies comparing indomethacin to other oral non-steroid anti-inflammatory drugs (NSAIDs). This study aims to address this knowledge gap by evaluating the incidence of post-ERCP pancreatitis in patients who received indomethacin compared to those who received alternative NSAIDs (acetic, phenyl acetic and propionic acid derivatives, coxibs, oxicams) within three days before the procedure.
Methods: We conducted a retrospective cohort study in the United States using the TriNetX database to identify adults (≥18 years) undergoing ERCP and treated with indomethacin, excluding those with gallstone pancreatitis. This cohort was matched with patients undergoing ERCP and treated with other NSAIDs intake using 1:1 propensity score matching. The primary outcome was the incidence of post-ERCP pancreatitis within 7 days after ERCP, while the secondary outcome was the 7-day mortality. With censoring applied, Kaplan-Meier analysis with hazard ratios (HRs) and 95% CIs were used to compare time-to-event rates at daily time intervals.
Results: A total of 49,375 patients undergoing ERCP and treated with indomethacin were identified and propensity matched with 15,310 patients with AUD treated with other NSAIDs, resulting in 14,716 matched pairs. Patients treated with indomethacin were older, and had lower rates of pancreatitis risk factors (p < 0.001) as compared to controls (Table 1). Patients undergoing ERCP who received indomethacin had no significant differences in the risk of post-ERCP pancreatitis (1.6% vs. 1.3%; adjusted hazard ratio (aHR) = 1.21, 95% CI: 0.98–1.41), and mortality (0.4% vs. 0.5%, aHR = 0.87, 95%CI: 0.62 – 1.21) at 1 week follow-up (Table 2).
Discussion: Our findings provide real-world evidence that rectal indomethacin use in patients undergoing ERCP does not significantly reduce the risk of post-ERCP pancreatitis or mortality compared to other NSAIDs. Further prospective studies are needed to evaluate the benefits of other NSAIDs in prevention on post-ERCP pancreatitis, specifically in high risk patients and cost effectiveness.

Figure: Baseline characteristics, data will be provided on sex/race/ethnicity/alcohol use, BMI and drugs: Non significant after matching

Figure: Incidence of post ERCP pancreatitis
Disclosures:
Pradeep Yarra indicated no relevant financial relationships.
Yassine Kilani indicated no relevant financial relationships.
Shravya Ginnaram indicated no relevant financial relationships.
Ahmad Basil Nasir indicated no relevant financial relationships.
Daniel Gonzalez Mosquera indicated no relevant financial relationships.
Alfred Nelson indicated no relevant financial relationships.
Wissam Kiwan indicated no relevant financial relationships.
Pradeep Yarra, MD1, Yassine Kilani, MD1, Shravya R. Ginnaram, MD2, Ahmad Basil Nasir, MD1, Daniel Alejandro. Gonzalez Mosquera, MD3, Alfred Nelson, MD1, Wissam Kiwan, MD1. P2234 - Indomethacin Does Not Reduce Risk of Post-ERCP Acute Pancreatitis Compared to Other NSAIDs: A Propensity Score-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
